CLINICIAN'S CAPSULE
What is known about this topic?
Several high-sensitivity troponin (hs-cTn) algorithms to rule out myocardial infarction (MI) exist, leaving physicians unsure which to implement.
What did this study ask?
This study prospectively compares the diagnostic performance of 1- and 2-hour hs-cTn algorithms for Canadian emergency department patients with chest pain.
What did this study find?
Both algorithms were accurate at diagnosing and excluding MI, but the 2-hour algorithm may offer several practical advantages.
Why does this study matter to clinicians?
Institutions implementing hs-cTn assays can choose between 1- or 2-hour algorithms, which can safely expedite patient care.
INTRODUCTION
Chest pain and symptoms of suspected cardiac ischemia lead to millions of emergency department (ED) visits annually worldwide.Reference Reichlin, Hochholzer and Bassetti1 Research has demonstrated that very low concentrations of high-sensitivity cardiac troponin (hs-cTn) sampled on ED arrival, especially in combination with a non-ischemic electrocardiogram (ECG), are highly sensitive for index myocardial infarction (MI).Reference Pickering, Than and Cullen2,Reference Andruchow, Boyne and Innes3 However, guidelines recommend a single hs-cTn testing strategy only for patients with at least 3-hours since symptom onset given the risk of false-negative results in early presenters.Reference Roffi, Patrono and Collet4 Because the majority of patients will not meet these stringent criteria, serial hs-cTn sampling is recommended for most patients. Several rapid diagnostic algorithms measuring small but clinically significant changes in hs-cTn over fixed time intervals (usually 1 or 2 hours) have been validated, and while they are highly sensitive for index MI, they are less sensitive for 30-day major adverse cardiac events (MACE).Reference Reichlin, Schindler and Drexler5–Reference Neumann, Twerenbold and Ojeda19
While European Society of Cardiology (ESC) 2015 guidelines endorse a 1-hour hs-cTn algorithm,Reference Roffi, Patrono and Collet4 concerns about the optimal resampling interval and what interval change in hs-cTn concentrations is clinically meaningful persist.Reference Body and Carlton20 Moreover, few studies have directly compared the performance of these algorithms to each other within the same patient cohort. A recent publication examined the diagnostic performance of 14 rule-out MI algorithms,Reference Wildi, Boeddinghaus and Nestelberger18 including the ESC 1-hour and Reichlin 2-hourReference Reichlin, Cullen and Parsonage8 high-sensitivity cardiac troponin-T (hs-cTnT) algorithms, but did not compare their rule-in performance. Finally, the bulk of research to date has been performed in Europe and Australasia with samples processed in a single core laboratory likely representing optimal test conditions and may not be reflective of real-world assay performance. Consequently, with several rapid diagnostic algorithms to choose from, selecting the optimal algorithm balancing ED length of stay, patient safety, and logistical considerations has become a challenge for many Canadian EDs implementing hs-cTn assays.
The objective of this study is to prospectively validate and compare the ESC-endorsed 1-hour rapid diagnostic algorithm using hs-cTnTReference Roffi, Patrono and Collet4 with a 2-hour hs-cTnT algorithmReference Reichlin, Cullen and Parsonage8 (Figure 1) under real world conditions by quantifying their diagnostic performance for index MI and 30-day MACE (sensitivity, specificity, negative and positive predictive values [NPV/PPV]), and negative and positive likelihood ratios [(LR+/LR-]). Our hypothesis is that both the 1- and 2-hour algorithms will have similar diagnostic accuracy for both index MI and 30-day MACE.
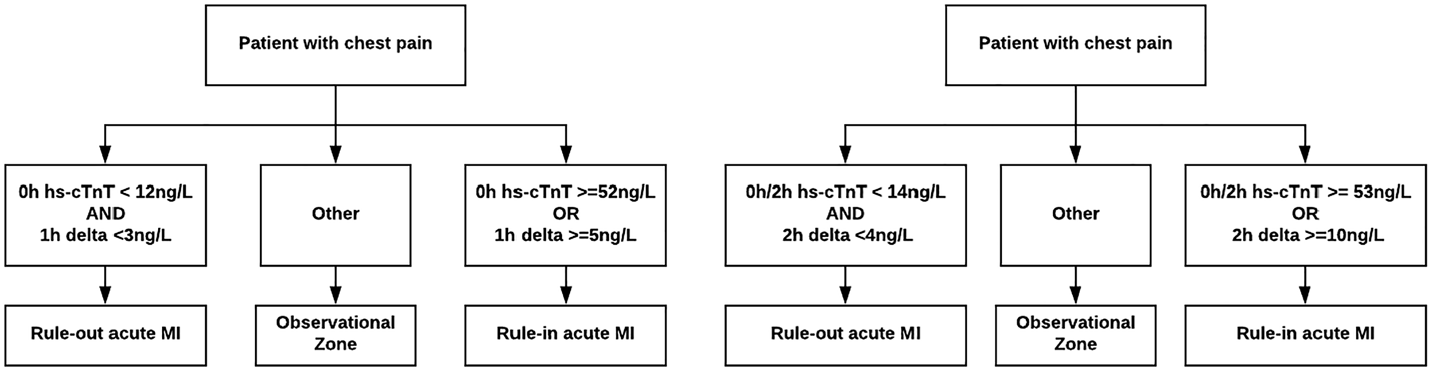
Figure 1. One and 2-hour rapid diagnostic algorithms using hs-cTnT.
METHODS
Study design, time period, and setting
This prospective observational cohort study was conducted at a large urban level one trauma and regional percutaneous coronary intervention (PCI) centre in Calgary, Alberta, from August 2014 to September 2016. The ED has an annual patient volume of approximately 80,000 visits, including approximately 2,500 annual visits for chest pain, and is staffed exclusively by certified emergency physicians. This study was conducted according to the Standards for Reporting Diagnostic accuracy studies (STARD) guidelines for studies of diagnostic accuracy (Supplement) and was approved by the University of Calgary Conjoint Health Research Ethics Board.
Population
Patients were eligible if they were ages 25 years and older, presented to the ED with Canadian Emergency Department Information System (CEDIS) standardized chief complaintsReference Grafstein, Bullard, Warren and Unger21 of “chest pain – cardiac features” or “cardiac type pain” and required serial troponin testing to rule out MI at the discretion of the attending emergency physician. Patients were excluded from the study if, according to the attending emergency physician, they had ST-elevation MI, clear acute ischemic changes, or new arrhythmia (not including sinus tachycardia, premature atrial contractions, premature ventricular contractions, paced rhythm, or rate-controlled atrial fibrillation/atrial flutter) on the initial ECG, were diagnosed with an acute coronary syndrome in the 30 days prior to the index visit, were hemodynamically unstable, had advanced renal failure requiring dialysis, or were unable to provide consent secondary to language barriers or cognitive issues. Patients unable to have valid samples collected within the +/− 30-minute window of the specified collection time were excluded from the analysis.
Troponin assay
Hs-cTnT (Roche Elecsys® High-sensitivity, 5th generation, Troponin T assay performed on the cobas e 601 instrument as per the manufacturer's specifications) results were obtained for all patients. This assay has a limit of blank (LoB) of 3 ng/L, a limit of detection (LoD) of 5 ng/L, a 99th percentile of 14 ng/L in a healthy population, and an imprecision corresponding to a 10% coefficient of variation at the limit of quantitation (LoQ) of 13 ng/L.
Study procedures
Trained research assistants approached consecutive patients between 0800 and 2000 hours, 7 days a week, to obtain written informed consent and collect demographic data. Attending ED physicians used standardized case report forms to collate detailed clinical information regarding patient presentation and past medical history. All patients consented for a 30-day telephone follow-up and detailed review of medical records. Presenting (0-hour) hs-cTnT samples were collected as part of routine care by an emergency physician order or as part of a nurse-initiated chest pain protocol; care providers were not blinded to these results. After enrolment, 1- and 2-hour research hs-cTnT samples were collected by either a trained phlebotomist or registered nurse; these results were not disclosed to care providers. If an emergency physician wished to obtain 1- or 2-hour hs-cTnT results for a study patient, a separate physician order was required.
All patients underwent a detailed review of medical records incorporating the 30-day period following the index visit. Outcome data were also obtained using hospital administrative databases, Alberta vital statistics, and the APPROACH registry. APPROACH is a registry that prospectively collects data on all patients admitted with a cardiac diagnosis or who have a revascularization procedure in the province of Alberta.Reference Ghali and Knudtson22
Outcomes
The primary outcome was index MI diagnosed on the basis of a rise and/or fall of hs-cTnT above the 99th percentile in the appropriate clinical context, in accordance with the Third Universal Definition of Myocardial Infarction.Reference Thygesen, Alpert and Jaffe23 The secondary outcome was 30-day MACE (including MI, revascularization, or cardiac death) and its individual components. Cardiac death was adjudicated in accordance with the American College of Cardiology/American Heart Association 2014 Definitions for Cardiovascular Endpoints.Reference Hicks, Tcheng and Bozkurt24 All outcomes were independently adjudicated by two physicians (board-certified cardiologist and board-certified emergency physician) after the review of all available clinical information, including ECGs, troponin results, imaging findings, and clinical documentation. All disagreements were resolved by consensus.
Data analysis and sample size
Descriptive statistics were performed for the cohort. Sensitivity, specificity, NPV, PPV, and LR+/LR- with 95% confidence intervals were calculated for the 1- and 2-hour algorithms. A pre-specified sensitivity analysis was conducted to examine the impact of excluding patients with ischemic ECG findings on outcome prevalence. Statistical analyses were performed using R Version 3.2.3 (www.r-project.org ). To obtain a 95% confidence interval of +/-1.0% for the outcome of 30-day MI (estimated prevalence 2%), a sample size of 753 patients was calculated. The two-proportion z-test was used to compare test characteristics between algorithms.
RESULTS
A total of 1,167 eligible patients with at least one hs-cTnT sample collected were enrolled as part of a related study examining hs-cTnT concentrations on presentation (0-hour), which has been published separately.Reference Andruchow, Boyne and Innes3 Of these, 559 patients were excluded because they did not require serial troponin sampling to rule out MI in the opinion of the attending emergency physician (usually because of prolonged and/or atypical symptoms), leaving 608 patients eligible for this study. The final data set included 350 patients with valid 1-hour and 550 patients with valid 2-hour hs-cTnT samples (Figure 2). Samples for the 1-hour cohort and 2-hour cohort were collected on average 7.4 minutes (SD 7.3 minutes) and 6.8 minutes (SD 7.1 minutes) from the specified collection time, respectively. Only 46 (13.1%) of 1-hour and 66 (12.0%) of 2-hour samples were collected more than 15 minutes from the designated collection time. Patient baseline characteristics and 30-day outcomes were similar among the two cohorts (Table 1). No patients were lost to follow-up.
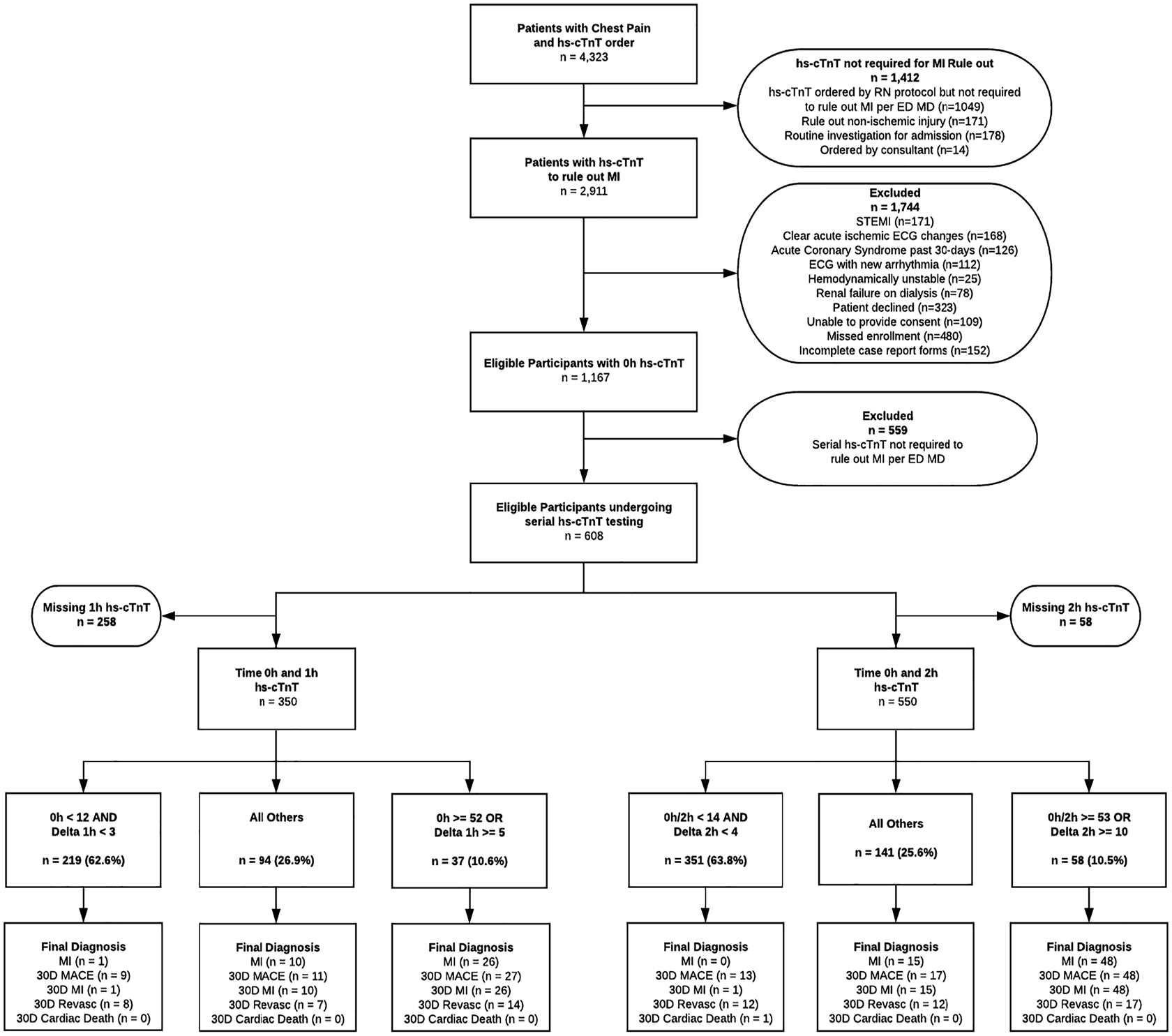
Figure 2. Standards for Reporting Diagnostic accuracy studies (STARD) diagram.
Table 1. Patient characteristics and outcomes
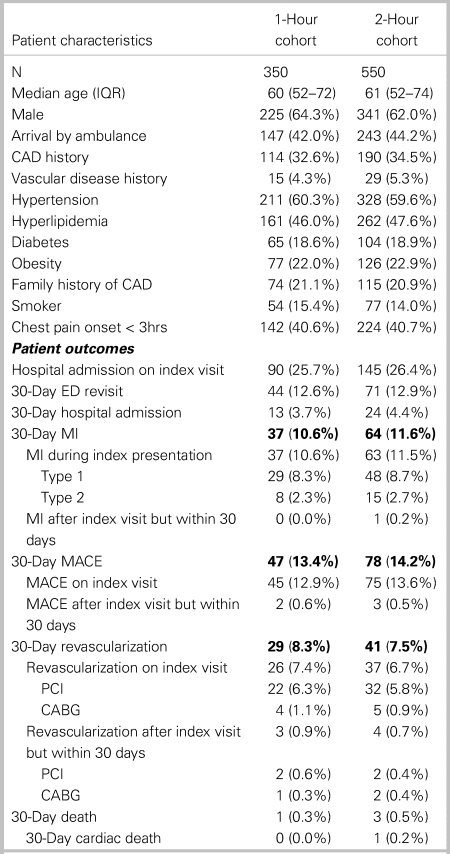
CABG = coronary artery bypass graft; CAD = coronary artery disease; ED = emergency department; IQR = interquartile range; MACE = major adverse cardiac event; MI = myocardial infarction; PCI = percutaneous coronary intervention.
The 1- and 2-hour algorithms categorized similar proportions of patients as low risk, 62.6% v. 63.8%, respectively (Table 2). However, whereas the low-risk criteria of 2-hour algorithm were 100% sensitive (95% CI: 91.6–100%) capturing all 48 MI patients, the 1-hour algorithm missed 1 of 37 patients with MI on the index visit (sensitivity 97.3%, 95% CI: 85.8–99.9%). Sensitivity for 30-day MACE was lower for both algorithms, with 9 of 47 patients with MACE missed by the 1-hour algorithm (sensitivity 80.9%, 95% CI: 66.7–90.9%) and 13 of 78 patients with MACE missed by the 2-hour algorithm (sensitivity 83.3%, 95% CI: 73.2–90.8%). Both the 1- and 2-hour algorithms missed one patient with 30-day MI. One patient with 30-day cardiac death was missed by the 2-hour algorithm, whereas no cardiac deaths were missed by the 1-hour algorithm. None of these differences were statistically significant (two-proportion z-test > 0.05 in all cases).
Table 2. Comparison of 1- and 2-hour algorithms low-risk criteria
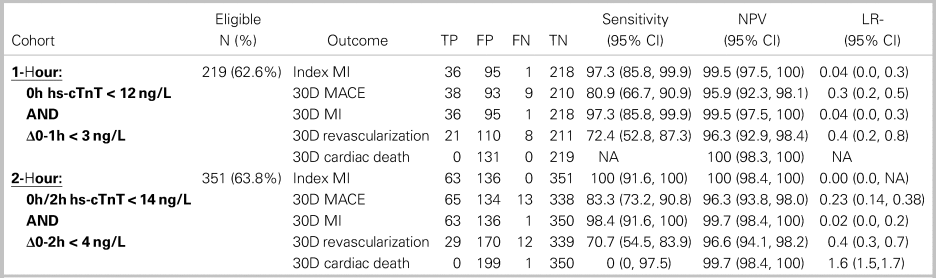
TP = true positive; FP = false positive; FN = false negative; TN = true negative; NPV = negative predictive value; LR- = negative likelihood ratio.
Both the 1-hour and 2-hour hs-cTnT algorithms categorized similar proportions of patients as high risk (10.6% v. 10.5%) and were highly specific for index MI and 30-day MACE (Table 3). While the PPV point estimate for the 2-hour algorithm for index MI was higher (82.8% v. 70.3%), this difference was not statistically significant (two-proportion z-test, z = 1.43, p = 0.1527). Both algorithms classified about one-quarter of patients in a non-diagnostic observational zone with an ~11% index MI and ~12% 30-day MACE prevalence (see Figure 1).
Table 3. Comparison of 1- and 2-hour algorithms high-risk criteria
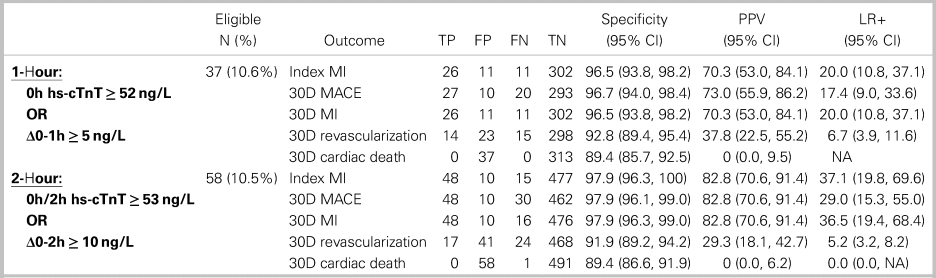
TP = true positive; FP = false positive; FN = false negative; TN = true negative; PPV = positive predictive value; LR+ = positive likelihood ratio.
DISCUSSION
Interpretation of findings
Both algorithms were highly accurate for both ruling-in and ruling-out MI. The 1-hour algorithm had a sensitivity of 97.3% and -LR of 0.04 for both index and 30-day MI. The 2-hour algorithm had 100% sensitivity and -LR of 0.00 for index MI and 98.4% sensitivity and -LR of 0.04 for 30-day MI. These findings suggest that the low-risk criteria of both algorithms confidently rule out MI. Similarly, the high-risk criteria of both algorithms were highly specific for index MI and 30-day MACE, with + LRs ranging from 17.4 to 37.1. These findings suggest that false-positive diagnoses of MI using either algorithm are unlikely. Patients with high-risk hs-cTnT findings should receive immediate treatment and cardiology consultation in the appropriate clinical context.
Not surprisingly, both algorithms were less sensitive for 30-day MACE, emphasizing the continued importance of ECG findings and thorough clinical assessment, in addition to biomarkers in identifying patients at risk of short-term MACE. However, given NPVs for 30-day MACE of ~96% for both algorithms, in the absence of high-risk clinical features, discharge with outpatient follow-up appears safe for the majority of patients with low-risk hs-cTnT results.
Comparison to previous studies
To our knowledge, this is only the second direct comparison of the ESC-recommended 1-hour rapid diagnostic algorithm with a 2-hour hs-cTnT algorithm in the same cohort.Reference Wildi, Boeddinghaus and Nestelberger18 These results validate prior workReference Reichlin, Schindler and Drexler5,Reference Reichlin, Twerenbold and Wildi7,Reference McRae, Innes and Graham16,Reference Wildi, Boeddinghaus and Nestelberger18 and confirm that both algorithms can rapidly rule out MI and facilitate early discharge by identifying almost two-thirds of patients as low risk for 30-day MACE. The widespread adoption of similar algorithms across Canadian EDs could reduce ED length of stay, defer testing of low-risk patients to community settings, and decongest EDs and inpatient units, a finding which has already been demonstrated in Europe.Reference Twerenbold, Jaeger and Rubini Gimenez25
While no statistically significant differences in diagnostic test characteristics for any outcomes were observed between the algorithms in our study, the diagnostic accuracy point estimates for the 2-hour algorithm were consistently better than those of the 1-hour algorithm. A similar pattern was recently observed in a recent large comparative analysis.Reference Wildi, Boeddinghaus and Nestelberger18 We believe that the superior point estimates for the 2-hour algorithm are observed because the 2-hour algorithm uses larger serial change (delta) values, making it less vulnerable to misclassification owing simply to analytic variability. The lack of a statistically significant difference in the sensitivity of the 2-hour algorithm compared with that of the 1-hour algorithm observed in this study is likely a function of an overall small sample size.
Analytic variability arises primarily from two sources: pre-analytical variation (relating to issues occurring prior to a sample analysis that can affect results, including test-ordering, patient preparation, specimen collection, processing, and storage) and analytical variation (relating to inherent inaccuracies of the assay itself).Reference Nichols and Clark26 Suppose a real-world analytical variability of +/-2 ng/L per sample. This is much less likely to result in misclassification using the 2-hour algorithm rule-out delta of < 4 ng/L and a rule-in delta of ≥ 10 ng/L than the 1-hour algorithm rule-out delta of < 3 ng/L and a rule-in delta of ≥ 5 ng/L (see Figure 1). In fact, to improve rule-in specificity, other authors have proposed even much larger rule-in delta cutoffs using the same hs-cTnT assay (≥ 16 ng/L on repeat samples within a 24-hour period).Reference Douville and Thériault27
Strengths and limitations
Strengths of this study include prospective data collection, a relevant patient population (ED patients requiring MI rule-out with serial troponin testing in the opinion of an emergency physician), care provider blinding to 1- and 2-hour hs-cTnT sample results, comprehensive follow-up, two-physician outcome adjudication, and conduct in real-world clinical and laboratory settings.
The primary limitation of this study is the small sample size, which limits the precision of the estimates that can be generated from it. We were unable to achieve our desired sample size owing largely to local practice patterns that included discharging almost half of patients after a single hs-cTnT assay. Furthermore, logistical issues, including an ethics requirement for physician assessment prior to collecting research samples, made it challenging to collect appropriately timed samples, particularly for 1-hour samples, with 200 fewer being collected. This may have led to selection bias, as patients with higher risk presentations may have been assessed more urgently by a physician and thus more likely to be enrolled in the study. However, the practice of assessing higher risk patients more quickly (making them more likely to be successfully enrolled) would result in higher risk patients being concentrated in the study cohorts. The fact that these criteria performed well despite this adds strength to our results.
The prevalence of index MI (~11%) in these cohorts is lower than the original derivation and validation studies, which ranged between 16% and 17%,Reference Reichlin, Schindler and Drexler5,Reference Reichlin, Twerenbold and Wildi7,Reference Reichlin, Cullen and Parsonage8 likely owing to the exclusion of patients with acute ischemic ECG changes. Sensitivity analysis reveals that if all 168 patients with acute ischemic ECG changes had been included in the 2-hour cohort and diagnosed with index MI, the prevalence of index MI could have been as high as 32.2%. However, because these patients clearly represent a high-risk subgroup, clinical practice would dictate that, even in the presence of normal serial hs-cTnT concentrations, most are likely to be admitted for further evaluation. Our focus on patients without ischemic ECG changes allows an evaluation of algorithm performance in those patients who specifically need troponin testing to diagnose or rule out MI.
Finally, patients with potential alternative presentations of cardiac ischemia (e.g., dyspnea, weakness, back pain, nausea, and abdominal pain) were not included, and it is possible that this systematically underrepresents women, patients with diabetes, elderly patients, and other subgroups who are less likely to report chest pain. However, requiring a chief symptom of chest pain as one of the primary enrolment criteria has been commonplace in the MI diagnostic literature and may prevent dilution of disease prevalence in the cohort when presentations unlikely to be cardiac are included.
Clinical implications
Based on these data and prior literature, both 1- and 2-hour hs-cTnT algorithms are highly accurate for ruling-in and ruling-out MI in patients with suspected ischemic chest pain. We strongly encourage the adoption of similar hs-cTn algorithms in EDs across Canada given their proven performance and ability to expedite care and improve the objectivity of evaluation. We believe that 2-hour algorithms offer several practical advantages, given potential challenges collecting appropriately timed 1-hour samples in busy EDs, and because the larger serial change (delta) cutoffs are less susceptible to misclassification secondary to analytic variability. Therefore, we believe that it is most prudent for centres implementing new hs-cTn assays to consider 2-hour algorithms, which, while still facilitating rapid decision-making, may be more practical to implement and offer a greater margin of safety – an opinion that is shared by other authors.Reference Vasile and Jaffe28,Reference Andruchow, Kavsak and McRae29
Research implications
Despite the excellent performance of the low- and high-risk criteria for both algorithms, approximately one-quarter of patients remain in a non-diagnostic “observational zone” after serial troponin testing. Because this cohort has an ~11% MI prevalence, careful clinical assessment is required to ensure a safe disposition. To date, there are no clear guidelines on how best to manage these patients; however, recommendations for additional serial hs-cTnT sampling to assess for ongoing myocardial injury, careful consideration of alternative diagnoses, and cautious disposition using a validated risk prediction tool, such as the HEART score, would seem prudent.Reference Andruchow, Kavsak and McRae29 Validating an evidence-based pathway to safely disposition observational zone patients should be a priority for future research.
CONCLUSIONS
Both the ESC 1-hour algorithm and an alternative 2-hour hs-cTnT diagnostic algorithm can rapidly and accurately rule-in or rule-out MI for about three-quarters of ED patients with chest pain. While no statistically significant differences in diagnostic performance were found between the algorithms, we note the 2-hour algorithm to offer several practical advantages and may be easier to implement in everyday practice. Implementation of hs-cTn algorithms has the potential to significantly decrease ED length of stay and resource utilization. Future research should focus on further comparative analysis of rapid diagnostic algorithms in real-world practice and providing more objective guidance for the observational zone population with indeterminate hs-cTn results.
Supplementary material
The supplemental material for this article can be found at https://doi.org/10.1017/cem.2020.349.
Acknowledgements
We would like to acknowledge the assistance of our research team, including Heidi Boyda, Katrina Koger, and Tiffany Junghans in the completion of this study.
Competing interests
None declared.
Financial support
This research was funded by an investigator-initiated, unrestricted research grant from Roche Diagnostics Canada. None of the study investigators received any direct or indirect compensation for the conduct of this study.









