Article contents
Factors governing the formation of lithiophorite at atmospheric pressure
Published online by Cambridge University Press: 01 January 2024
Abstract
Lithiophorite is a naturally occurring Mn oxide mineral commonly found in soils and sediments. The usual method of synthesizing lithiophorite is via a hydrothermal process in an autoclave at relatively high temperature and pressure. In the present study, an alternative, reflux method, at atmospheric pressure, for synthesis of lithiophorite was developed successfully. The influence of reaction duration, temperature, type of precursor birnessite (H-birnessite, Na-birnessite, aged Na-birnessite), and pH on the formation of lithiophorite were investigated by reflux treatment of lithium-aluminum hydroxide complex ion (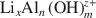 )-exchanged birnessite. The results show that the degree of conversion of lithiophorite decreases with decreasing reaction temperature. Lithiophorite can be obtained at pH values from 5.0 to 9.0, but a circumneutral pH is more favorable for formation at atmospheric pressure. Conversion of Na-birnessite (Bir-OH) to lithiophorite is more favored than aged Na-birnessite (Bir-OH-A). Lithiophorite was not obtained by refluxing the
)-exchanged birnessite. The results show that the degree of conversion of lithiophorite decreases with decreasing reaction temperature. Lithiophorite can be obtained at pH values from 5.0 to 9.0, but a circumneutral pH is more favorable for formation at atmospheric pressure. Conversion of Na-birnessite (Bir-OH) to lithiophorite is more favored than aged Na-birnessite (Bir-OH-A). Lithiophorite was not obtained by refluxing the 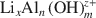 ion-exchanged H-birnessite (Bir-H) sample. The rate of conversion of lithiophorite increases with increasing reflux time. Lithiophorite synthesized by a reflux process has pseudo-hexagonal crystals of 0.1–0.5 µm with a chemical composition of Li0.24Al0.46MnO2.67(H2O)1.25. The results have important implications for the origin and underlying mechanism of lithiophorite formation in the environment.
ion-exchanged H-birnessite (Bir-H) sample. The rate of conversion of lithiophorite increases with increasing reflux time. Lithiophorite synthesized by a reflux process has pseudo-hexagonal crystals of 0.1–0.5 µm with a chemical composition of Li0.24Al0.46MnO2.67(H2O)1.25. The results have important implications for the origin and underlying mechanism of lithiophorite formation in the environment.
- Type
- Article
- Information
- Copyright
- Copyright © The Clay Minerals Society 2009
References
- 10
- Cited by




