Article contents
Partitioning of Fe(II) in reduced nontronite (NAu-2) to reactive sites: Reactivity in terms of Tc(VII) reduction
Published online by Cambridge University Press: 01 January 2024
Abstract
Clay minerals impart important chemical properties to soils, in part, by virtue of changes in the redox state of Fe in their crystal structures. Therefore, measurement of Fe(III)/Fe(II) and partitioning of Fe(II) in different reactive sites in clay minerals (during biological and chemical Fe(III) reduction) is essential to understand their role and their relative reactivity in terms of reduction and immobilization of heavy metal contaminants such as technetium. This study had three objectives: (1) to understand the degree of dissolution of nontronite (Fe-rich smectite) as a result of chemical and biological reduction of Fe(III) in the structure; (2) to quantify partitioning of chemically and biologically produced Fe(II) into different reactive sites in reduced nontronite, including aqueous Fe2+, ammonium chloride-extractable Fe(II) (mainly from the ion-exchangeable sites, denoted as Fe(II)NH4Cl${\rm{Fe}}{\left( {{\rm{II}}} \right)_{{\rm{N}}{{\rm{H}}_4}{\rm{Cl}}}}$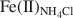 ), sodium acetate-extractable Fe(II) (mainly from the surface complexation sites, denoted as Fe(II)acetate), and structural Fe(II) (denoted as Fe(II)str); and (3) to evaluate the reactivity of these Fe(II) species in terms of Tc(VII) reduction. Chemical and biological reduction of Fe(III) in nontronite (NAu-2) was performed, and reduced nontronite samples with different extents of Fe(III) reduction (1.2–71%) were prepared. The extent of reductive dissolution was measured as a function of the extent of Fe(III) reduction. Our results demonstrated that chemically and biologically produced Fe(II) in NAu-2 may be accommodated in the NAu-2 structure if the extent of Fe(III) reduction is small (< ∼30%). When the extent of reduction was >∼30%, dissolution of nontronite occurred with a corresponding decrease in crystallinity of residual nontronite. The Fe(II) produced was available for partitioning into four species: Fe(ab)2+${\rm{Fe}}_{\left( {{\rm{ab}}} \right)}^{2 + }$
), sodium acetate-extractable Fe(II) (mainly from the surface complexation sites, denoted as Fe(II)acetate), and structural Fe(II) (denoted as Fe(II)str); and (3) to evaluate the reactivity of these Fe(II) species in terms of Tc(VII) reduction. Chemical and biological reduction of Fe(III) in nontronite (NAu-2) was performed, and reduced nontronite samples with different extents of Fe(III) reduction (1.2–71%) were prepared. The extent of reductive dissolution was measured as a function of the extent of Fe(III) reduction. Our results demonstrated that chemically and biologically produced Fe(II) in NAu-2 may be accommodated in the NAu-2 structure if the extent of Fe(III) reduction is small (< ∼30%). When the extent of reduction was >∼30%, dissolution of nontronite occurred with a corresponding decrease in crystallinity of residual nontronite. The Fe(II) produced was available for partitioning into four species: Fe(ab)2+${\rm{Fe}}_{\left( {{\rm{ab}}} \right)}^{2 + }$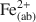 , Fe(II)acetate, Fe(II)NH4Cl${\rm{Fe}}{\left( {{\rm{II}}} \right)_{{\rm{N}}{{\rm{H}}_4}{\rm{Cl}}}}$
, Fe(II)acetate, Fe(II)NH4Cl${\rm{Fe}}{\left( {{\rm{II}}} \right)_{{\rm{N}}{{\rm{H}}_4}{\rm{Cl}}}}$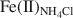 , and Fe(II)str. The increase in Fe(II)acetate during the early stages of Fe(III) reduction indicated that the Fe(II) released had the greatest affinity for the surface-complexation sites, but this site had a limited capacity (∼60 µmol of Fe(II)/g of NAu-2). The subsequent increase in Fe(II)NH4Cl${\rm{Fe}}{\left( {{\rm{II}}} \right)_{{\rm{N}}{{\rm{H}}_4}{\rm{Cl}}}}$
, and Fe(II)str. The increase in Fe(II)acetate during the early stages of Fe(III) reduction indicated that the Fe(II) released had the greatest affinity for the surface-complexation sites, but this site had a limited capacity (∼60 µmol of Fe(II)/g of NAu-2). The subsequent increase in Fe(II)NH4Cl${\rm{Fe}}{\left( {{\rm{II}}} \right)_{{\rm{N}}{{\rm{H}}_4}{\rm{Cl}}}}$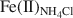 indicated that the released Fe(II) partitioned into the exchangeable sites once the amount of Fe at the surface-complexation sites reached half of its maximum site capacity. The fraction of Fe(II)str decreased concomitantly, as a result of Fe(II) release from the NAu-2 structure, from 100% when the extent of Fe(III) reduction was <30% to nearly 65% when the extent of Fe(III) reduction reached 71%. The Fe(II)acetate and Fe(II)str exhibited greater reactivity in terms of Tc(VII) reduction than the Fe(II)NH4Cl${\rm{Fe}}{\left( {{\rm{II}}} \right)_{{\rm{N}}{{\rm{H}}_4}{\rm{Cl}}}}$
indicated that the released Fe(II) partitioned into the exchangeable sites once the amount of Fe at the surface-complexation sites reached half of its maximum site capacity. The fraction of Fe(II)str decreased concomitantly, as a result of Fe(II) release from the NAu-2 structure, from 100% when the extent of Fe(III) reduction was <30% to nearly 65% when the extent of Fe(III) reduction reached 71%. The Fe(II)acetate and Fe(II)str exhibited greater reactivity in terms of Tc(VII) reduction than the Fe(II)NH4Cl${\rm{Fe}}{\left( {{\rm{II}}} \right)_{{\rm{N}}{{\rm{H}}_4}{\rm{Cl}}}}$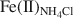 . Clearly, the surface-complexed and structural Fe(II) are the desirable species when reduced clay minerals are used to reduce and immobilize soluble heavy metals in contaminated groundwater and soils. These results have important implications for understanding microbe—clay mineral interactions and heavy metal immobilization in clay-rich natural environments.
. Clearly, the surface-complexed and structural Fe(II) are the desirable species when reduced clay minerals are used to reduce and immobilize soluble heavy metals in contaminated groundwater and soils. These results have important implications for understanding microbe—clay mineral interactions and heavy metal immobilization in clay-rich natural environments.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2008, The Clay Minerals Society
References
- 62
- Cited by




