Introduction
After widespread implementation of antiretroviral therapy (ART), several studies from European countries have reported very low mother-to-child transmission (MTCT) rates of HIV [Reference Reitter1–Reference Warszawski4]. Despite these successes, approximately 200–300 HIV-positive children are reported to the European Centre for Disease Prevention and Control (ECDC) annually. Approximately half of these children are immigrants and are, most probably, infected already in their country of origin [5]. To confirm MTCT as the route of transmission, one should know the mother's HIV status at the time of the delivery. This is, unfortunately, known in only a limited number of cases.
The proportion of immigrants of all HIV-positive patients is increasing in Europe. There is a concern that eliminating MTCT is less successful among immigrant women, e.g. a Canadian study showed that immigrant HIV-positive women received less adequate prenatal care compared with Canadian women [Reference Ng6]. Likewise there appears to be barriers to testing and care also in Europe [Reference Hernando7].
Elimination of MTCT requires a bundle of interventions: ART to the mother and the newborn, appropriate management of delivery and avoidance of breastfeeding. Since only diagnosed women can be treated, the cornerstone in preventing MTCT is timely HIV diagnosis. Women should be diagnosed in early antenatal care if not diagnosed before conception.
When all precautions are met, even zero transmissions can be achieved in a subgroup of women on ART at the time of conception, as reported by Mandelbrot et al. [Reference Mandelbrot8]. However, we are unaware of data on eliminating MTCT on a national level nor on women delivering with undiagnosed HIV.
Finland implemented a universal opt-out antenatal HIV screening in 1998. Several cities had already screened pregnant women for some years. Approximately 98% of all pregnant women in Finland attend this screening [Reference Surcel9]. With the approval of ethical committees for research purposes, the health care registers in Finland permit individual identification of all HIV-positive women giving birth either before or after their own HIV diagnosis.
The aim of the main study was to determine the national MTCT rate in Finland since the beginning of the HIV epidemic. We also evaluated the risk factors leading to late diagnosis of HIV during pregnancy and compared the diagnostics and treatment results between natives and immigrants. As a substudy, we reviewed women that delivered <2 years before their HIV diagnosis, analysed the HIV status of these children and sought to clarify the reasons leading to unknown HIV status before the delivery.
Methods
In the main study, we included all HIV-positive women with at least one delivery in Finland during 1983–2013 after receiving HIV diagnosis and all children born in these deliveries. In a substudy, we included women who had a delivery within 2 years prior to HIV diagnosis and whose HIV diagnosis was unknown at the time of the delivery. The year 1983 appeared to be the starting point as the first year a woman was diagnosed HIV-positive in Finland [10]. To allow adequate follow-up of the children, we chose 31 December 2013 as the closing point.
In Finland, each individual receives a unique, 10-digit personal identification number at birth or immigration by the Civil Registration System. With this number, a person can be identified in different registers and hospitals’ medical records throughout the country.
Laboratories report each individual's first positive HIV antibody test result to the National Infectious Diseases Register maintained by the National Institute of Health and Welfare [10]. Physicians report detailed information on the mode of transmission of HIV, nationality, country of transmission and stage of the disease to the registry.
Hospitals report all children born in Finland to the Medical Birth Register regardless of mother's nationality [11]. This register contains information on the mother, the delivery and the newborn from 1987 onwards. We collected the treatment/delivery data from 1983 to 1987 from the hospitals’ medical records.
The Finnish Maternity Cohort Register contains information on antenatal infection screening results from 1998 onwards [12] and was used to complete and verify the data.
HIV-positive patients are treated in Infectious Diseases Clinics in 20 hospitals in Finland; 16 of them also manage deliveries of HIV-positive women.
We combined the National Infectious Diseases Register with Medical Birth Register and Finnish Maternity Cohort Register to identify all HIV-positive women ever delivering in Finland. Their children were identified from these registries. Data on women and children were collected from the registers as well as from the hospitals’ medical records (Fig. 1). The study subjects were not contacted.

Fig. 1. Combining the registers to identify all HIV-related pregnancies and the HIV status of the children. Two hundred and twelve women were diagnosed HIV-positive before or during the pregnancy. Twelve women were diagnosed HIV-positive <2 years after a delivery with an unknown HIV status at the time of the delivery.
We extracted the following data on women of known HIV diagnosis at the time of the delivery: year, country, site of HIV diagnosis, mode of transmission, possible previous AIDS defining illnesses, hepatitis B surface antigen (HBsAg) status, hepatitis C antibodies and number of children and induced abortions prior to HIV diagnosis, as well as country of origin and year of immigration. We collected the following data for each pregnancy: ART status before the pregnancy and during each trimester, CD4 lymphocyte count at the beginning of the pregnancy, HIV viral load (VL) preceding the delivery, HIV status of the newborn and information on previous children born in Finland and their HIV status.
For women who had given birth within 2 years prior to their own HIV diagnosis, we collected the following data: year and hospital of the delivery, country of origin, time of the HIV diagnosis related to the delivery and possible explanation of why HIV test was not taken before the delivery. For children born before the mother's HIV diagnosis, only HIV status was collected.
The ethics committee of Helsinki University Hospital approved the study (344/13/03/00/2014). The National Institute of Health and Welfare granted permission to combine the registers and to perform the nationwide study (THL/1535/6.02.00/2015). All 16 participating hospitals provided local permissions to carry out the study and to use their medical records. According to the Finnish legislation, informed consent is not required for this type of a retrospective study.
Statistical analysis
HIV diagnosis was classified as before pregnancy if the diagnosis had been made before conception. HIV diagnosis after gestational week (GW) 20 was considered very late as was the initiation of ART after GW 24.
The detection limit of VL measurement declined during the study years from 1000 to 20 copies/mL. We grouped all undetectable VL results with detectable VL <200 copies/mL as a good treatment response. Detectable VL exceeding 200 copies/mL preceding the delivery was considered an inadequate treatment response.
For comparisons between the groups, χ 2 and Fisher's exact test were used for categorical variables and the non-parametric Mann–Whitney U-test for continuous variables. We used univariate and multivariate logistic regression models to evaluate risk factors for HIV diagnosis during the pregnancy as compared with diagnosis before the pregnancy.
IBM SPSS version 21.0 (Chicago, IL, USA) was used in all statistical analyses.
Results
During 1983–2013, altogether 212 women with HIV diagnosis gave birth to 290 children. Helsinki University Hospital accounted for 65.2% of these deliveries. The first delivery occurred in 1993. The prevalence of diagnosed HIV among parturients increased from 3.1/100 000 in 1993 to 65.8/100 00 in 2013.
The population of 212 HIV-positive women consisted of altogether 138 (65.1%) immigrants and 74 (34.9%) native women. Before the index delivery, 38.2% (n = 81) of the women had children and 30.7% (n = 65) had undergone an induced abortion. The proportion of women with previous children was higher in immigrants compared with natives (44.2% vs. 27.0%, P = 0.014). After the HIV diagnosis, 36.8% (n = 78) of the population had more than one child with no difference between immigrants and natives (Table 1).
Table 1. Demographics of the 212 women delivering at least one child after HIV diagnosis 1983–2013

IQR, interquartile range; IDU, intravenous drug use; MTCT, mother-to-child HIV transmission; GW, gestational week.
Before the index delivery, 3.8% had a preceding AIDS-defining illness, 4.7% were HBsAg-positive and 10.8% hepatitis C virus-antibody positive. After the index delivery, there were no cases of new AIDS-defining illnesses or hepatitis seroconversions.
The proportion of immigrants among the HIV-positive parturients increased significantly from 18.2% before 1999 to 75.3% during 2011–2013 (P < 0.001). The highest increase was found in women born in sub-Saharan Africa and in Eastern Europe (Fig. 2).
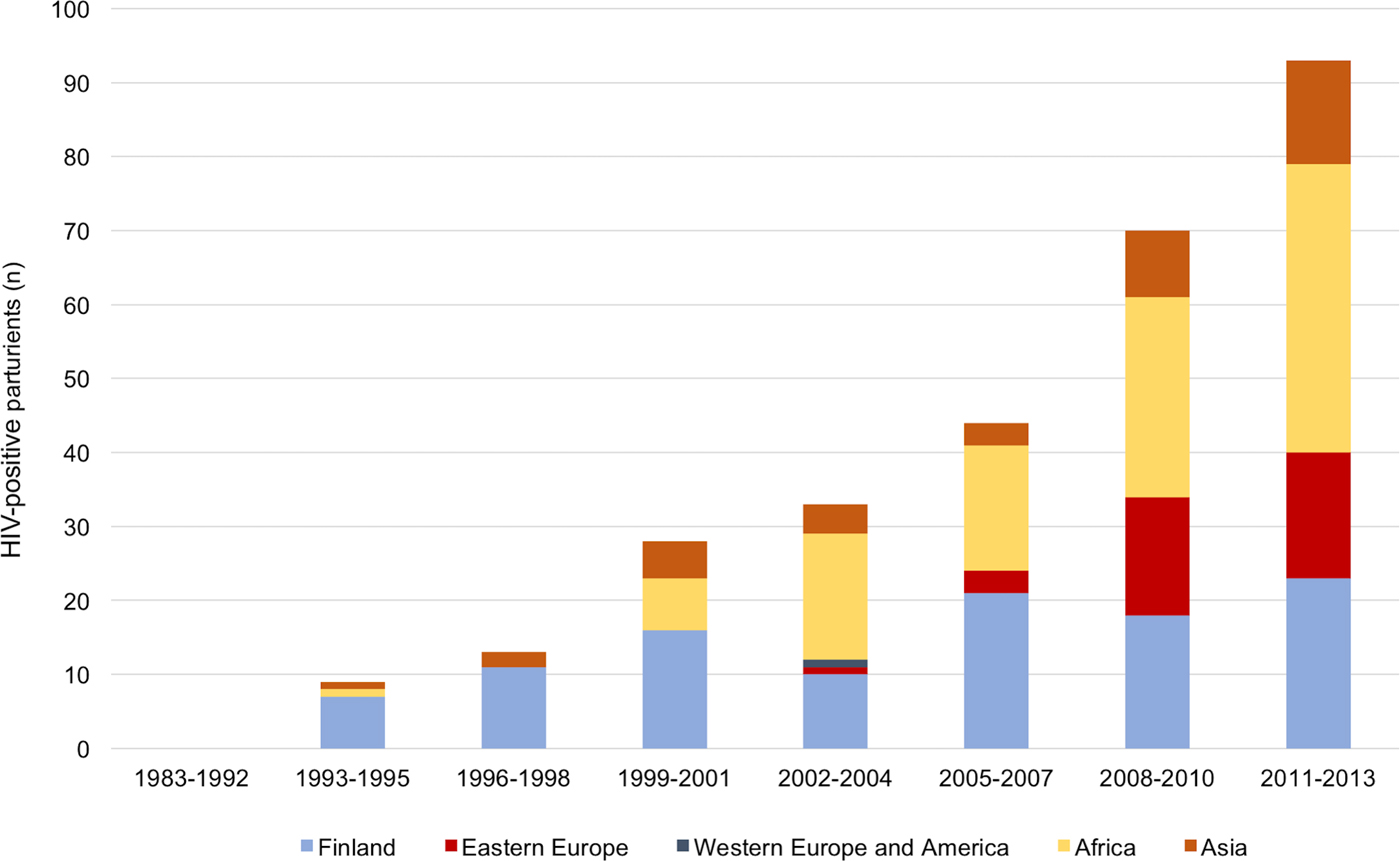
Fig. 2. The number and the origin of the parturients according to the year of delivery. The number of immigrants among HIV-positive parturients increased over fourfold between 1999 and 2013.
Factors associated with HIV diagnosis during the pregnancy
When analysing the risk factors for HIV diagnosis during pregnancy, we included only the first pregnancy as HIV-positive. Of all 212 women, 45.8% were diagnosed during pregnancy. If all 290 pregnancies were included, the proportion of women diagnosed during the pregnancy decreased to 34.1%. The proportion of women diagnosed during the pregnancy did not change with time nor did the proportion diagnosed very late after GW 20.
The HIV diagnosis at the antenatal screening programme was more common outside the Helsinki metropolitan area than in Helsinki (65.2% vs. 35.8%, P < 0.001) (Fig. 3). The proportion of immigrant and native women diagnosed during pregnancy did not differ significantly (53.0% vs. 42.3%, P = 0.153).

Fig. 3. Site of diagnosis in the Helsinki metropolitan area and outside the metropolitan area. Altogether 46% of the parturients were diagnosed during pregnancy with a significant difference between Helsinki metropolitan area and the rest of Finland.
In multivariate analyses, women aged over 30 years, heterosexual route of transmission compared with intravenous drug use, living outside of the Helsinki metropolitan area and being of Eastern European origin were risk factors for being diagnosed during pregnancy (Table 2). When assessing the risk factors for very late diagnosis, immigrants were more often diagnosed after 20 GWs compared with natives (13.0% vs. 2.7%, P = 0.014) and with CD4 counts <200 cells/μL (16.4% vs. 1.5%, P = 0.002). When a higher CD4 cut-off of 350 cells/μL was used, immigrants and natives did not differ significantly (45.0% vs. 29.6%, P = 0.176).
Table 2. Risk factors for not being diagnosed before the pregnancy for women diagnosed in Finland (N = 186)

OR, odds ratio; CI, confidence interval; IDU, intravenous drug use.
a Natives and one immigrant of Western European origin were combined as Western European origin.
Antiretroviral treatment
If assessing all 290 pregnancies, 53.9% of mothers were on ART at the time of conception with no difference between immigrants and natives (55.6% vs. 51.4%, P = 0.570). The proportion of women on ART at the time of conception was higher in 2010–2013 compared with earlier years (54.4% vs.45.6%, P = 0.001).
An increasing proportion of women were on ART by GW 24 (Fig. 4) over time with no difference between immigrants and natives (74.5% vs. 73.6%, P = 0.870). All women were prescribed ART in the third trimester. Six women refused to start ART with no difference between immigrants and natives.

Fig. 4. The proportion of the parturients on antiretroviral therapy (ART) during the second trimester (bars) and with HIV viral load (VL) <200 copies/mL preceding the delivery (line) over the study period.
The proportion of women with a good treatment response to ART (VL preceding the delivery undetectable or <200 copies/ml) increased during the study period (Fig. 4). We found no difference in the proportion of women with a good treatment response to ART between immigrants and natives (85.9% vs. 82.1%, P = 0.390), or between the Helsinki metropolitan area and the rest of Finland (84.1% vs. 85.1%, P = 0.819).
MTCT of HIV during 1983–2013
Of the 290 children born to mothers with diagnosed HIV infection, none were infected.
During the study period, altogether 12 pregnant women had an unknown HIV status during the delivery and were diagnosed within 2 years afterwards (Fig. 1). Eight deliveries occurred before implementation of the national screening programme in 1998, three immigrant women did not attend the screening and one native woman was infected during the pregnancy after a negative test result in early pregnancy. Three (i.e. 25%) children of these undiagnosed mothers were infected, the last one in 2000.
Discussion
The present study shows that elimination of MTCT of HIV is feasible in a low-prevalence, high-resource country. No child has been infected perinatally with HIV in Finland since the year 2000, even though the number of deliveries in HIV-positive women has increased and a high proportion (46%) of them continue to be diagnosed first during pregnancy. Moreover, the proportion of HIV diagnoses made during pregnancy did not decrease.
Older age, living outside the Helsinki area, Eastern European origin and not being infected by drug use were associated with an increased risk of being diagnosed only during pregnancy. Intravenous drug users in Finland are actively offered low-threshold HIV testing and the infections have been diagnosed early [Reference Kivela13], which probably explains this surprising result concerning drug use in preventing late diagnosis. Moreover, most women of Eastern European and South-East Asian origin arrive in Finland to work, or as spouses, and thus are not tested routinely, unlike refugees and asylum seekers.
Several recent publications report a substantially lower number of mothers diagnosed with HIV in the pregnancy, for example, from Denmark (18%), Germany (24%) and the UK (28%) [Reference Reitter1, Reference Townsend3, Reference Orbaek14], but in these articles, all pregnancies were included and multiparity obviously decreases the figures. We only included each woman's first pregnancy after the HIV diagnosis in our analyses, considering this the most relevant. In our study, the rate of diagnosis during pregnancy was 34% if all pregnancies were included; this is still higher than published elsewhere. Favarato et al. concluded that pregnancy is an important opportunity to migrant women to learn their HIV status [Reference Favarato15]. Our study in a very-low-prevalence setting extends this conclusion also to local women, since both groups were equally undiagnosed before the pregnancy. About half of the women diagnosed at antenatal screening had no obvious risk factor for HIV infection and could have been left out in a risk-based screening. Before the implementation of the universal antenatal screening in 1998, the mother was undiagnosed in 8/30 (27%) of HIV-related deliveries. The same was true in only 4/272 (1.5%) deliveries after 1998. Taken together, these observations highlight the continuous need for systematic antenatal HIV screening, even in low-prevalence countries.
Notably, 30% of women had had an induced abortion before the first pregnancy as HIV-positive. Although we were unable to specify whether they were already HIV-positive at the time of the abortion, it could serve as a valuable testing opportunity.
Treatment of HIV is not centralised in Finland, but the Helsinki area accounted for 65% of HIV-related pregnancies. Women living outside the Helsinki metropolitan area were more often diagnosed late than women living in the Helsinki area. There was no difference, however, in treatment response between Helsinki and the other areas, even the smallest hospitals. This is reassuring, since long geographical distances hinder centralisation of HIV care. Earlier studies have shown discrepant findings in this respect: an increased risk of late diagnosis in people living in low-prevalence areas was shown in non-pregnant patients in Spain and the UK [Reference Castilla16, Reference Chadborn17]. In contrast, in a recent study from the UK, women living outside the London area had lower odds on late booking to antenatal care compared with women living in London [Reference French18]. This study describes the delays in diagnostics and treatment initiation, but lacks information on treatment response.
The proportion of immigrants among HIV-positive parturients increased in our study although later and to a lesser extent than in many other European countries [Reference Favarato15]. Women originating from sub-Saharan Africa formed the largest group, but the proportions of women from South-East Asia and Eastern Europe were substantial, unlike in many other countries.
The proportion of women with very late diagnosis (after GW 20) and with very low CD4 counts (<200 cells/μL) at diagnosis were significantly higher among immigrants than natives. The difference in very late diagnosis was mainly driven by women from sub-Saharan Africa arriving in Finland late in pregnancy. Special multidisciplinary attention should be taken to reach these women as soon as possible after their immigration. Our figures of very late diagnosis were nonetheless substantially lower than in a recent European cohort study [Reference Favarato15]. In that study, immigrants were more often diagnosed during pregnancy, diagnosed late in pregnancy and with a lower CD4 count than local women. In our study, the proportions of diagnosis during earlier in the pregnancy, ART coverage during the second trimester, virological treatment results and proportion on ART at the time of conception were similar between these groups. In a recent study from the UK [Reference French18], immigrants, especially from sub-Saharan Africa, booked late for an antenatal visit and those newly diagnosed started ART later than natives, which is in contrast to our results.
In our study, only half of the previously diagnosed women were on ART at the time of conception with no differences between immigrants and natives. Most of the study period occurred before the 2012 WHO recommendation that women should stay on ART after the delivery [19]. The proportion on ART increased worldwide after this recommendation and has further increased after the results of the START study [Reference Lundgren20] showed ART to be beneficial to all HIV-positive individuals, regardless of the CD4 count. According to present guidelines, all women are recommended to continue lifelong ART after the delivery. This may reduce the number of loss to follow-up, since not being on ART seems to increase that risk [Reference Gerver21].
Most women, whether diagnosed before or during the pregnancy, were on ART by GW 24, the proportion reaching 87% after 2008. A great majority of women achieved good virological response, similar to most recently published studies [Reference Reitter1, Reference Townsend3, Reference Orbaek14]. Even though ART is free of charge for all pregnant women, not everyone agreed to start it, which leaves their children with an excess risk of transmission. The hesitation of these women should be addressed as early as possible with a multidisciplinary approach.
The generalisability of our study results is limited by the relatively low number of HIV-related pregnancies in Finland, even when taking the first 30 years of the epidemic into account. Due to the retrospective nature, we were able to use only data collected at the time. However, we believe our data are complete, since we were able to identify all HIV-related pregnancies by combining the nationwide registers, and including pregnancies and their outcomes prior to women's own HIV diagnosis. In addition, we had access to actual patients’ medical records, so we were not dependent on the information collected by the registers only.
By limiting the data collection to 2 years prior to each woman's HIV diagnosis, we might have underestimated the number of HIV-related pregnancies. Since the Medical Birth Register starts from 1987, we may have missed a HIV-related pregnancy during 1983–1987, with 11 women diagnosed during that time. Nevertheless, no HIV-positive children were born in Finland during those years. With a high level of certainty, we can be sure to have included all HIV-positive children born 1983–2013, since no new HIV diagnosis among children born in Finland have been reported by November 2017.
In conclusion, our study has demonstrated that national elimination of MTCT is feasible in a high-income, low-prevalence country. The mainstay of this success is to enable all women (and men) in their fertile age to know their HIV status in early antenatal screening – although preferably already before the conception – and to reassure HIV-positive women on the safety and effectiveness of ART also during pregnancy.
Acknowledgements
Members of the FINHIVPREG study team: Inka Aho, Helsinki University Hospital; Timo Hautala, Oulu University Hospital, Oulu; Taru Finnilä, Turku University Hospital, Turku; Hanna Viskari, Tampere University Hospital, Tampere; Janne Mikkola, Kanta-Häme Hospital District, Hämeenlinna; Sari Hämäläinen, Kuopio University Hospital, Kuopio; Tuomas Nieminen, Satakunta Central Hospital, Pori; Elina Kärnä, Seinäjoki Central Hospital, Seinäjoki; Ville Lehtinen, Päijät-Häme Central Hospital, Lahti; Maija Rummukainen, Central Finland Health Care District, Jyväskylä; Antti Väänänen, Lapland Central Hospital, Rovaniemi; Jukka Heikkinen, North Carelia Central Hospital, Joensuu; Risto Pietikäinen, Kymenlaakso Central Hospital, Kotka; Pekka Suomalainen, South Carelia Central Hospital, Lappeenranta; Sakari Vuorinen, Mikkeli Central Hospital, Mikkeli. The authors would like to thank Mikko Lehtovirta for the technical assistance with the figures.
Financial support
This work was supported by the Finnish Medical Association (IA) and Infektiotautien tutkimusyhdistys ry (IA).
Conflict of interest
None.











