Introduction
Immunisations are among the most successful and cost-effective public health interventions. Their use has led to the eradication of smallpox, regional elimination of polio, measles and rubella, and substantial reductions in the morbidity and mortality attributed to diphtheria, tetanus and pertussis. WHO estimates that between 2 and 3 million child deaths are prevented through vaccination annually [1]. In 2015 mortality rates of 134 000 due to measles and 34 000 due to tetanus were estimated [Reference Liu2]. This highlights how more deaths could be prevented through the optimal use of currently existing vaccines and the identification of populations where vaccination programmes have failed to reach. The success of routine and supplementary immunisation programmes has traditionally been measured by the coverage achieved with the third dose of diphtheria–tetanus–pertussis vaccine (DPT3) among children aged 12–23 months [Reference Burton3]. Detection of specific antibodies to tetanus can also be used as an indicator of vaccination with tetanus toxoid-containing vaccine since natural immunity following tetanus disease does not occur as the quantity of tetanus toxin required to produce disease is inadequate to induce an immune response. Similarly, vaccine coverage data for children aged 12–23 months has also been used to document the implementation of measles immunisation programmes. In contrast, tests for measles-specific antibodies detect antibodies resulting from both vaccine administration and wild-type infection.
In the Ugandan vaccination programme, tetanus vaccination is given as part of DPT-HepB + Hib. Three doses of tetanus toxoid-containing vaccine are given at 4-week intervals starting at 6 weeks of age. Females are given tetanus vaccination starting from 15 years of age and given a total of five doses in their lifetime. The need for further booster doses in childhood is recognised but not yet implemented. Measles vaccine is given at 9 months of age. Additional measles vaccination is given during supplemental immunisation campaigns. The last Measles SIAs were held in October 2015, targeting 6–59-month olds and an administrative coverage of 95% was obtained. Measles coverage was 97, 96 and 94% DPT3 97, 99 and 89% in 2013, 2014 and 2015, respectively [4]. Following the 2015 SIA, measles cases have significantly decreased, but have now increased with 9/54 as outbreaks investigated in 2017 confirmed as measles. Both programmes have had a major impact on disease burden [Reference Mbabazi5].
The gold standard methods for assessment of immunity to tetanus and to measles, which use serum samples, are by in vivo toxin neutralisation tests (TNT) using mice and in vitro plaque reduction neutralisation tests (PRNT), respectively [Reference Cohen6]. These tests are technically demanding and require specialised laboratory facilities. Consequently, relatively simpler tests such as enzyme immunoassay (EIA) are widely used to measure antibodies in serum to tetanus and to measles. Neutralisation and EIA tests for antibody are referred to as functional and non-functional, respectively. TNT measures the ability of antibodies in the test serum to complex with and neutralise a fixed lethal or paralytic dose of tetanus toxin when injected into mice. The neutralisation titre of test sera is compared with neutralisation observed with a reference International Standard serum. It is generally accepted that when measured by TNT, an anti-tetanus antibody level of 0.01 IU/ml is the minimum protective level. Because EIA methods detect both neutralising and non-neutralising antibody, the cut-off for the minimum level of protection against tetanus measured by standard EIA is usually considered to be between 0.1 and 0.15 IU/ml and this correlates well with results in TNT [Reference Simonsen7–Reference Borrow, Balmer and Roper9].
For measles, the gold standard test for antibody detection is the PRNT performed on serum samples, with an antibody level >120 mIU/ml determined to afford protection [Reference Chen10]. However, PRNT is costly and time-consuming and previous studies in some populations have shown that the qualitative measles EIA has a 99% positive predictive value for immunity [Reference Cohen, Doblas and Andrews11].
The reporting of immunisation coverage is a widely used programmatic tool, but it may not be an accurate estimation of true coverage. Recognised shortcomings of the accuracy of coverage data include settings where financial benefits may accrue through attaining a prescribed level of coverage or where vaccine potency may be impacted by inappropriate storage or cold chain. Serological methods to directly measure immunity to one or several of the vaccines administered are increasingly used and are useful validation tools. There are relatively little-published data on the antibody response to TT and duration of protection in routine vaccination programmes in low-income countries where the accelerated WHO schedule is used [Reference Scobie12]. As part of a study to evaluate the detection of anti-tetanus antibody using a non-invasive oral fluid sample, sera and vaccine histories were collected from a representative group of children aged 4 months to 6 years in Uganda. We report here the analysis of the levels of tetanus and measles antibodies by age following vaccination in this group of children.
Materials and methods
Subjects
Two hundred and six children; age range 12–75 months, were recruited at the Outpatient Department (OPD) of Entebbe Hospital. The children were recruited following description of the study to their parents/carers and signed informed consent obtained from attendees at the daily clinic over a 2-week period in August–September 2013. Children fulfilling study age criteria were recruited sequentially. Of the 206 OPD patients recruited, 205 reported verbally the receipt of at least one dose of DPT vaccine. One hundred and ninety-six of the OPD recruitees reported verbally having received measles vaccine. Only 106 of the OPD patients presented a vaccination card at the time of recruitment; 90 of whom had three documented doses of DPT, one who had received two doses of DPT and one having received a single dose of DPT. One hundred OPD recruitees had receipt of measles vaccine recorded on their vaccination card, 92 of which also included immunisation date. The second group of 113 children with ages ranging from 4 to 15 months was recruited from the Mother-Child Health Clinic (MCHC), Entebbe Hospital during September–October 2013. Of these children, 111 had vaccination cards with recorded dates of when the three DPT vaccine doses were administered. All 113 children had blood samples collected 3 or more weeks after receipt of DPT3. None of the 113 children recruited from the MCHC had a record of having received measles vaccine prior to specimen collection for this study. One hundred and eleven of the 113 children recruited at the MCHC were 9 months old or younger; that is, they were at or under the minimum recommended age (9 months) for receipt of measles vaccine in Uganda, at the time of sample collection. Both sets of children come from the same population served by Entebbe Hospital. The age distribution of the children recruited in the MCHC and the OPD is shown in Figure 1.
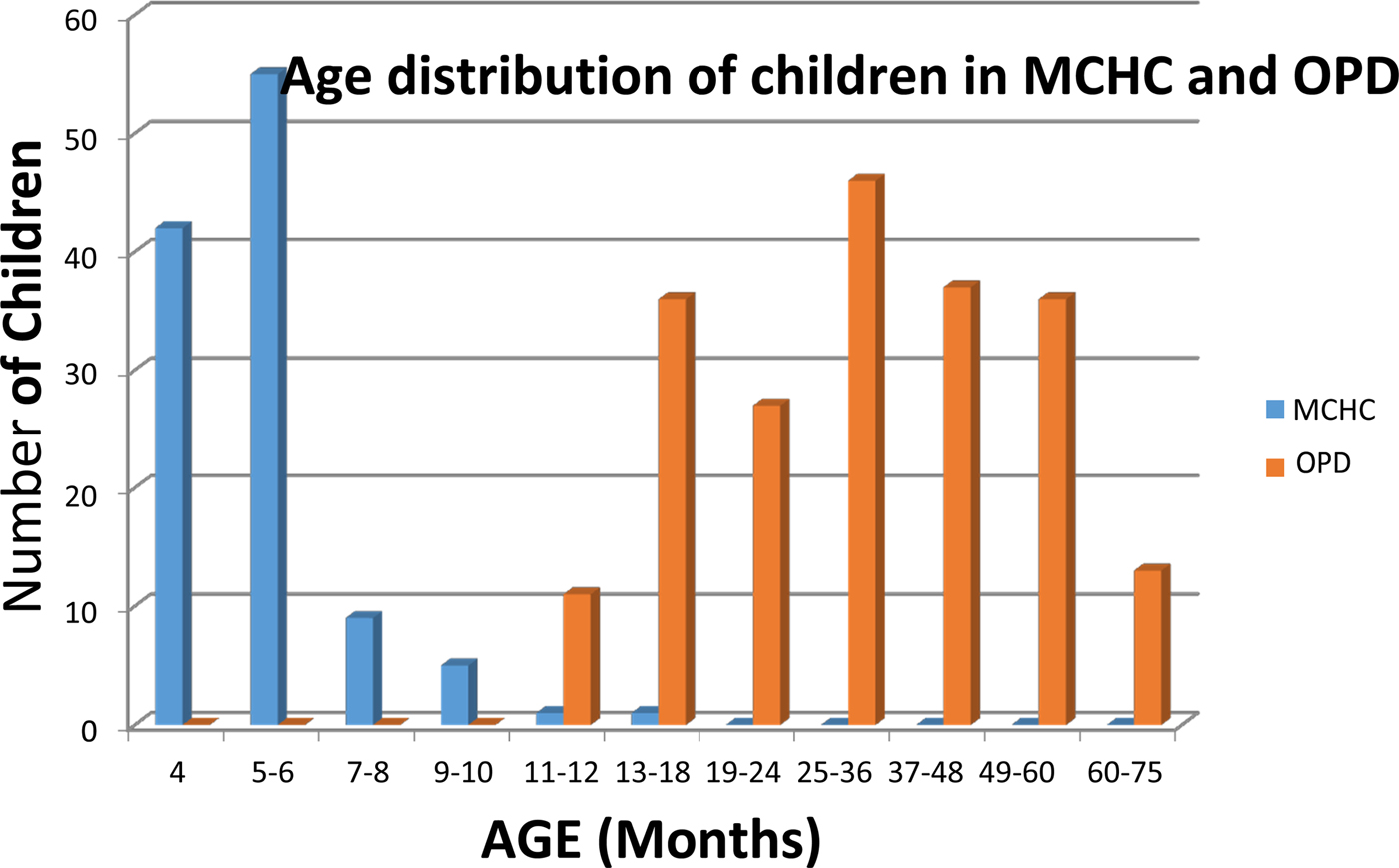
Fig. 1. Age distribution of children attending the Mother-Child Health Clinic (MHCH, shown in blue) and the Outpatients Department (shown in red) at Entebbe Hospital, Uganda.
Sera were separated from whole blood specimens at the Uganda Virus Research Institute (UVRI), aliquots dispensed into Sarstedt tubes and stored at −20 °C. The frozen aliquots were shipped under cold chain to Public Health England, UK for measles PRNT and EIA testing.
The study was conducted with ethical approval of the Uganda Virus Research Institute Research and Ethics Committee (UVRI, Ref: GC/127/13/06/05) and the Uganda National Council for Science and Technology (UNCST).
Antibody tests for measles and tetanus
Serum antibodies to measles were quantified by PRNT performed essentially as described by Cohen et al. (2007) [4] and by the Enzygnost anti-measles virus/IgG EIA kit (Siemens Healthcare, Frimley, UK). For measles PRNT, an antibody titre of ⩾120 mIU/ml was interpreted as conferring immunity [Reference Chen10].
The measles EIA was performed on a BEP III ELISA processor (Siemens Healthcare, Frimley, UK), according to the manufacturer's instructions. Primary serum dilutions were prepared manually and then transferred to the BEP III processor to complete the remaining stages of the assay. Both equivocal and positive Siemens EIA results were interpreted as being indicative of the presence of anti-measles antibody, as described elsewhere [Reference Cohen, Doblas and Andrews11].
Serum IgG antibodies to tetanus were quantified using the established Vacczyme Anti-Tetanus Toxoid IgG EIA Kit (The Binding Site Group Ltd, Birmingham, UK). The kit was calibrated by the manufacturer against the tetanus antitoxin reference preparation, 76/589, from the National Institute of Biological Standards and Control (NIBSC, Potters Bar, UK) it showed a high sensitivity (96.6%) in a recent comparative study [Reference van Hoeven13].
The VaccZyme Anti-Tetanus Toxoid IgG EIA was performed and results expressed as International Units per millilitre (IU/ml) as described in the manufacturer's instructions. For sera in which the concentration of anti-tetanus antibody was determined to be 0.15 IU/ml or greater, the result was interpreted as being protective against tetanus disease.
Timing of administration of DPT doses following the WHO accelerated schedule
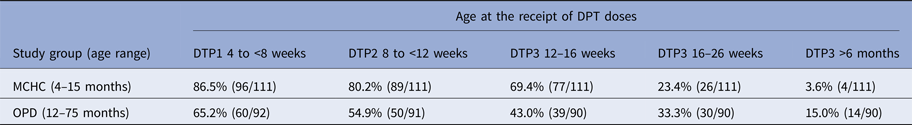
For children with documented evidence of receipt of three doses of DPT, the proportion receiving each dose of vaccine within a 4-week period spanning the WHO recommended schedule dates of 6, 10 and 14 weeks was collated for both the MCHC and OPD cohorts.
Statistical analysis – measles
Comparison of EIA and PRNT assays for measles antibody was performed by plotting paired results against one another and calculating the square of the correlation coefficient (R 2).
Statistical analysis – tetanus
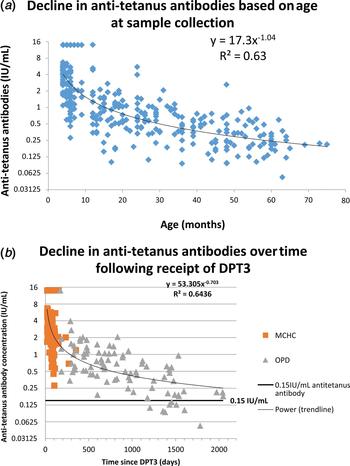
The anti-tetanus antibody titres for the 315 children with known ages were used in this study to model the decline in antibody concentration by age. The decline in antibody levels by age (a proxy for time since vaccination) was modelled using a power function which enabled a good fit to the data. This function uses a multiplicative scale for both antibody concentration and age. Comparison of titres in those aged greater and less than 1 was by a t test on log-transformed results.
The decline in antibody titre with time following DPT3 was analysed using a power function model, for the 111 children from the MCHC cohort and the 90 children from the OPD cohort for whom the dates of vaccine administration and specimen collection were available.
Results
Measles antibody results for children attending the MCHC aged 4–15 months and OPD aged 12–75 months
Ninety-six of the 113 children (85.0%), mostly under 6 months of age, had measles IgG titres below the protective level by PRNT (120 mIU/ml) (Table 1). Sera from 179 of the 203 (88.2%) children aged 12–75 months attending the OPD contained measles antibody titres >120 mIU/ml by PRNT. The sera were also tested for measles IgG antibody by EIA. Of the 196 children in both groups with PRNT titres >120 mIU/ml, 93.3% of children had detectable antibodies by EIA, i.e. either a positive (143/196) or equivocal (40/196) EIA result. Sera from 116/120 (96.7%) children with measles PRNT titres <120 mIU/ml were negative in the EIA. In those aged 4–9 months the median measles titre was <30 mIU/ml, the upper quartile 60 mIU/ml and maximum 921 mIU/ml. For those aged over 12 months the geometric mean was 644 mIU/ml, the median was 899 mIU/ml, interquartile range 355–1466 mIU/ml and range <30–14 505 mIU/ml
Table 1. Comparison of Siemen's measles IgG EIA and PRNT results for sera from the Mother-Child Health Clinic and the Outpatients Department, Entebbe Hospital
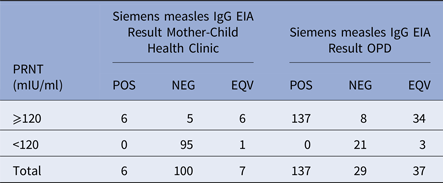
The sensitivity, specificity, positive and negative predictive value of the EIA compared with the PRNT, interpreting samples giving equivocal results in the EIA as positive were: 93.37% (95% CI 88.93–96.42), 96.67% (95% CI 91.69–99.08), 97.86% (95% CI 94.61–99.41) and 89.92% (95% CI 83.38–95.42)
The EIA and PRNT results showed a good correlation (R 2 = 0.83) (Figure 2). There was no detectable decline in antibody titres over the 12–75 months age range (data not shown).
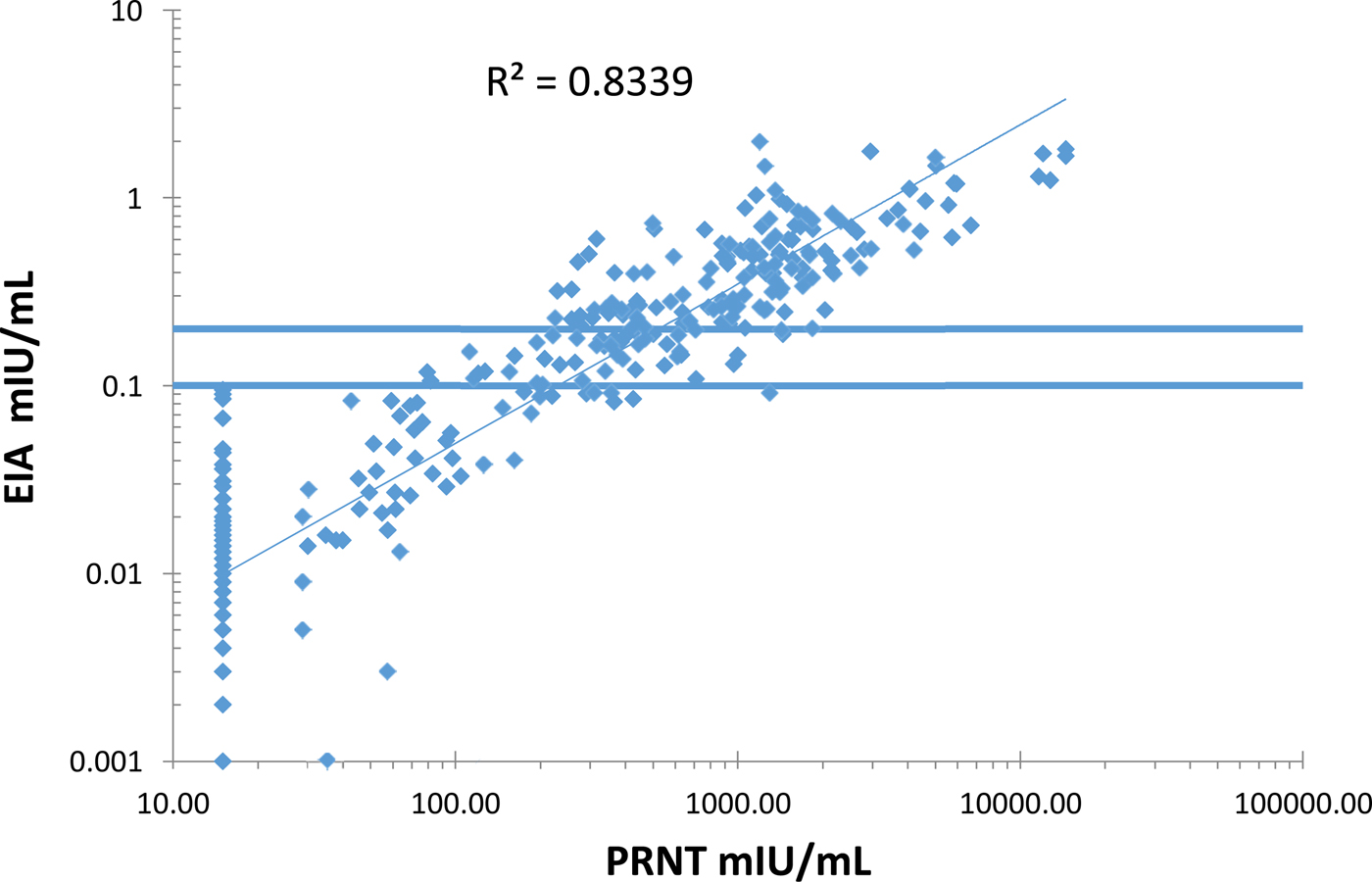
Fig. 2. Correlation between the measles plaque reduction neutralisation test (PRNT) and the Siemen's measles IgG EIA on serum samples from the Mother-Child Health Clinic and the Outpatients Department at Entebbe Hospital, Uganda.
Analysis of DPT vaccination timing
The WHO recommended schedule for DPT vaccine administration is 6, 10 and 14 weeks following birth. Analysis of the timing of administration of each of the three DPT vaccine doses in children with documented records of vaccination in the MCHC and the OPD group is shown in Table 2. Administration of vaccine within 2 weeks of the recommended schedule of 6, 10 and 14 weeks varied between the MCHC and the OPD groups. A greater proportion of children in the MCHC group, 69.4% received the three doses within the recommended schedule for vaccine administration, compared with 43.0% for the OPD group.
Table 2. Percentage of children receiving the first two doses of DTP within 2 weeks of the WHO recommended accelerated schedule and timing of receipt of DTP3
Tetanus antibody results in 203 children aged 12–75 months attending OPD and 113 children aged 4–15 month attending the MCHC
The distribution of serum anti-tetanus antibody titres in children measured by VaccZyme EIA is shown in Figure 3. All 113 children attending the MCHC had titres above the protective level of 0.15 IU/ml at the time of specimen collection; which ranged from 3 to 50 weeks (dates available for 111 children, geometric mean: 7.7 weeks) after the third dose of tetanus vaccine.
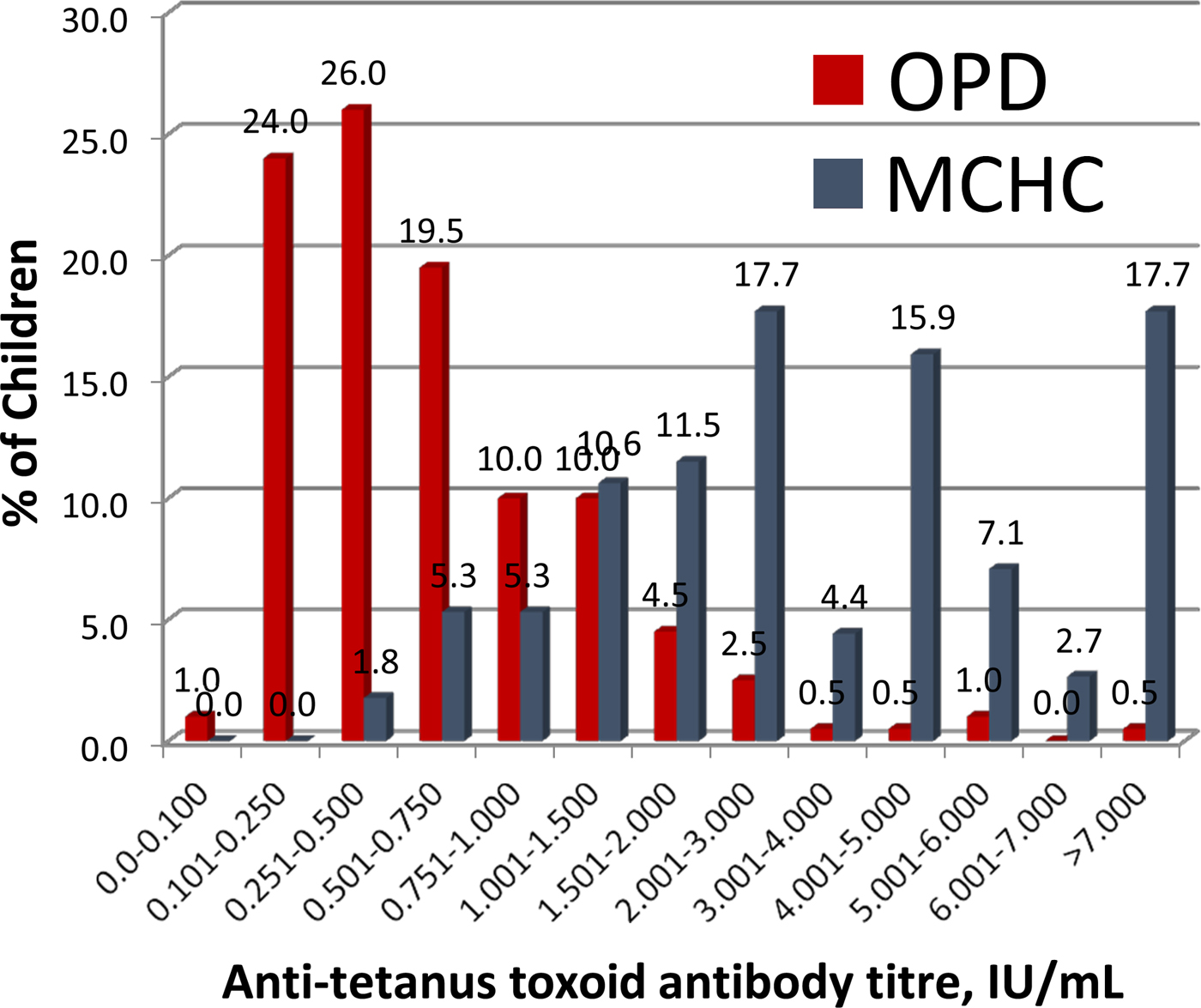
Fig. 3. Antitetanus toxoid antibody titres in serum samples from the Mother-Child Health Clinic (shown in blue) and the Outpatients Department (shown in red) at Entebbe Hospital, determined using the VaccZyme Tetanus toxoid IgG EIA (The Binding Site Group Ltd).
Ninety-three per cent (189/203) of children attending the OPD had serum anti-tetanus antibody titres >0.15 IU/ml. Parents or guardians of the 14 children with anti-tetanus antibody titres of <0.15 IU/ml all gave verbal recollection of DPT vaccine having been administered to the children. Nine of these 14 children had vaccination cards and eight (age range: 44–63 months) were recorded as having received all three doses of DPT vaccine before 6 months of age. Time of specimen collection from receipt of DPT3 ranged from 40 to 60 months for these eight children. One child, aged 27 months, was documented as having received only the first dose of DTP at 20 months of age. The five children without vaccination cards were aged 17–64 months.
Anti-tetanus antibody concentration and decline
The concentration of anti-tetanus antibody by age is shown in Figure 4a. The overall concentration of anti-tetanus antibodies was sixfold higher in children under 12 months; geometric mean concentration of 3.15 IU/ml, compared with 0.49 IU/ml for those older than 12 months (P < 0.001). Anti-tetanus antibody concentrations calculated from the power equation model demonstrate a decline from about 4 IU/ml at 4 months to 2 IU/ml at 8 months, 1 IU/ml at 16 months, 0.5 IU/ml at 32 months and 0.25 IU/ml at 64 months. This is a 50% decline as age doubles (Fig. 4). This shape of decline is characterised by an initial rapid fall followed by a more gradual decline and is commonly seen with post-vaccination antibody levels [Reference Borrow14].

Fig. 4. (a) The decline of antitetanus toxoid antibody titres with age determined using the VaccZyme Tetanus toxoid IgG EIA. (b) Decline of anti-tetanus toxoid antibody titres with time following the receipt of DPT3, determined using the VaccZyme Tetanus toxoid IgG EIA.
Decline in anti-tetanus antibody by time following receipt of DPT3 is shown in Figure 4b. Anti-tetanus antibody concentrations declined from about 5.1 IU/ml 4 weeks after vaccination to 3.1 IU/ml at 8 weeks, 1.4 IU/ml at 6 months, 0.8 IU/ml at a year and 0.3 by 4 years. This is a 39% decline in time since vaccination doubles.
Discussion
The measles antibody prevalence at 6 months of age is low. The prevalence is lower than other studies in communities before measles elimination and suggests other studies would be valuable. At this age, the antibody is likely to be of maternal origin and several factors could contribute to this low prevalence. One factor is that most mothers’ measles antibodies are due to vaccination in this population, which results in lower titres than from measles infection [Reference Itoh, Okuno and Hotta15]. The absence of boosting in this era of low measles incidence may also contribute to lower titres being passed on to their babies. Maternal health can also affect the transplacental transfer of antibody [Reference Palmeira16]. The result is of relevance to vaccination programmes, as this age cohort of susceptible individuals is consistent with the current epidemiology of measles in Uganda, where 22% of measles cases in 2014 occurred in children <9 months of age. This may enable vaccine to be given to younger children during outbreaks if this is indicated programmatically.
The measles antibody results in children aged 12–75 months are of interest and highlight the challenges of collecting accurate vaccine coverage data. One hundred and ninety-six parents gave a history of vaccination compared with only 100 who could produce a vaccination card whilst attending the clinic. If used to estimate vaccine coverage, these figures produce significant over ( + 9.5%) and underestimate (−44.1%), respectively, of the true prevalence of immunity in the population as assessed by PRNT. Although coverage studies are conducted in the community and enquire more deeply into vaccine history, they still rely on documentation and recollection. This does emphasise the potential value of serological tools to assess coverage, which is the overarching aim of this study. These results also support the wider use of population immunity studies to inform vaccine programmes, particularly where there are concerns about the accuracy of case-based surveillance. In this population, the Siemens’ EIA for measles antibody gave very similar results to PRNT suggesting it is suitable for seroepidemiological surveys.
Anti-tetanus antibody responses detected in children aged 4–15 months and in receipt of three doses of tetanus toxoid-containing vaccine were high and comparable with earlier studies [Reference Borrow, Balmer and Roper9]. In children aged 15–75 months an anti-tetanus antibody titre >0.15 IU/ml was maintained in 93% (189/203). This is consistent with previous data reported for children aged 1–5 years, in which the participants had received three doses of DPT according to the Expanded Programme on Immunisation, in the United Republic of Tanzania [Reference Aboud17]. Within our study cohort from Uganda, the proportion of children with vaccination cards containing documented dates for receipt of three DPT doses was higher in the younger MCHC group compared with the older OPD group reflecting the reason for attendance at the different clinics, routine vaccination is administered at the MCHC. The differences in timing of TT vaccine schedule between the two clinic populations was not investigated further but may reflect variations in programme performance over time. Serological testing demonstrated good vaccine coverage in both clinic populations.
There have been few studies of anti-tetanus antibody prevalence and persistence in young children in developing countries. In this study, a consistent fall in anti-tetanus antibody level was detected indicating that titres may drop below protective levels in childhood after three routine doses of DPT. This was the case for eight of 14 children (57%) in the OPD who had documented evidence of three DPT doses having been administered before 6 months of age and in whom the serum anti-tetanus antibody had fallen below the protective level, 3–5 years after receipt of the vaccine. This supports the need for further boosters after the age of 4–5 years, as recommended by WHO, which is not currently part of the programme in Uganda [18]. Complete and timely coverage of the vaccine schedule remain the key priorities for a successful vaccination programme.
Acknowledgements
The authors wish to acknowledge Desiree Barcinella for her excellent support in carrying out the measles PRNT. The study reported here was supported by the Bill and Melinda Gates Foundation, Global Development (Grant number OPP1066619), for a project entitled Measles, Tetanus toxoid, Immunoglobulin G (MTI) lateral flow device for rapid immunity assessment.
Conflict of interest
No conflicts of interest to report. No interests to declare.