Article contents
Antimicrobial hydrogels with controllable mechanical properties for biomedical application
Published online by Cambridge University Press: 07 May 2019
Abstract
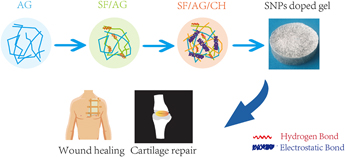
The antibacterial hydrogels can be widely used in the biomedical area owing to their excellent properties. The main limitation of antibacterial hydrogels is their poor mechanical strength. In this study, the novel hydrogels were fabricated with a mixture of silk fibroin (SF), chitosan (CH), agarose (AG), and silver nanoparticles (SNPs) via facile reaction condition without inorganic substances. The mechanical property of these fabricated hydrogels can be modulated by the concentration of SF or AG. The rheological studies demonstrated enhanced elasticity of CH-doped hydrogels. Because of the presence of CH and Ag in hydrogels, the antimicrobial property against gram-positive and gram-negative bacteria was exhibited. Cytocompatibility test proved the very low toxic nature of the hydrogels. In addition, these composite hydrogels have a smaller porosity, higher swelling ratio, and good compatibility, indicating their great potential for biomedical application.
Keywords
- Type
- Article
- Information
- Journal of Materials Research , Volume 34 , Issue 11: Focus Issue: (Nano)materials for Biomedical Applications , 14 June 2019 , pp. 1911 - 1921
- Copyright
- Copyright © Materials Research Society 2019
References
- 8
- Cited by




