Article contents
Density functional theory study on the adsorption of H, OH, and CO and coadsorption of CO with H/OH on the Pt2Ru3 surfaces
Published online by Cambridge University Press: 30 August 2016
Abstract
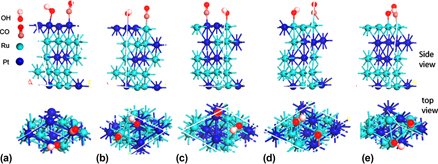
This paper presents a periodic density functional theory study on the adsorption of H, CO, and OH on Pt2Ru3 alloy surfaces containing different conformations of Pt and Ru atoms. The results show that for separate adsorption, H is preferentially adsorbed at Pt sites, whereas CO and OH are preferentially adsorbed at Ru sites. The adsorption strengths of H, CO, and OH are affected by ratio of the alloying atoms in top surface, the nature of the neighboring atom nearest to the adsorption site, and the conformation of alloying atoms in subsurface. We also investigated the coadsorption of CO with OH and the coadsorption of CO with H and found that the Pt–CO bond strength weakens. We also uncovered some information about the competitive adsorption behavior of adsorbates (CO, OH) with the aim of designing CO-tolerant Pt–Ru alloy catalysts.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 7
- Cited by





