Introduction
Monoon liukiuense (Hatus.) B. Xue et R.M.K. Saunders (previously called Polyalthia liukiuensis Hatus.) is a tree species belonging to the family Annonaceae (Hatusima Reference Hatusima1979, Xue et al. Reference Xue, Su, Thomas and Saunders2012). It grows on Iriomote Island (24.3°N, 123.7°E) and Hateruma Island (24.1°N, 123.7°E) in the Ryukyu (Nansei) Islands, Japan, and on Orchid Island (Lanyu, 22.0°N, 121.5°E) in Taiwan (Figure S1a). This species is distributed in the northernmost end of the distribution range of the other Monoon and Polyalthia species. Its habitats on all three islands are fragmented regenerating native forests surrounded by human-altered areas, such as rice paddies and sugarcane fields (Shinzato et al. Reference Shinzato, Yasuda, Kashima and Yokota2018). Because of these threats to its existence, M. liukiuense is classified as a critically endangered species on the Red List of Japan (Ministry of the Environment, Japan 2020). Furthermore, two habitat fragments, one on Iriomote Island and the other on Hateruma Island, have been designated as natural heritage sites of Taketomi Town, Japan (Taketomi Town Reference Town2018). Both the Ministry of Environment and the Taketomi town government require the conservation of this rare tree species (Ministry of the Environment, Japan 2020, Taketomi Town Reference Town2018).
To conserve a plant species, it is not only essential to preserve its present habitat but also to maintain its regeneration processes, which are often highly species-specific (Saeki et al. Reference Saeki, Yokogawa, Sashimura, Ashizawa, Ohtani, Kawano, Akashi and Furumoto2013). Seed dispersal, for instance, is a crucial process in the reproductive cycle of plants (Wang & Smith Reference Wang and Smith2002). Therefore, a conservation project should focus on preserving suitable areas and maintaining relationships between seed dispersers and plants.
M. liukiuense grows only in small areas, but the matured trees produce abundant fruit crops and many seedlings and saplings thrive under the fruiting trees (Figure S1b, c). A decrease in seed dispersal agents results in impacts on seed dispersal and an increased accumulation of seedlings under their parents (Howe & Miriti Reference Howe and Miriti2004). However, the seed disperser assemblage of M. liukiuense has yet been elucidated.
In this study, we hypothesised that M. liukiuense has lost effective seed dispersers in its present habitats. To test this hypothesis, fruit fate and frugivores were observed both in the canopy and on the ground of the habitats of M. liukiuense on Iriomote Island, Japan.
Materials and methods
Study site
The study was conducted on Iriomote Island (289.6 km2, Figure S1a), Japan. Based on the meteorological data from 1991 to 2020 (Japan Meteorological Agency 2020), the average annual temperature of this region was 23.9 °C (ranging between 15.8 to 32.2 °C), the monthly rainfall ranged from approximately 120 to 265 mm, and the annual average precipitation was 2025 mm. On Iriomote Island, M. liukiuense inhabits the limestone cliffs between rice paddies and sugarcane fields at approximately 20 m above sea level.
Study species
M. liukiuense is an evergreen arboreal species that grows to approximately 15 m tall (Ohashi Reference Ohashi, Ohashi, Kadota, Murata, Yonekura and Kihara2015). The intact ripe fruits are oblong drupes: 2.3–4.0 cm long, 1.6–2.6 cm wide, and the weight of the fresh fruit ranges between 3.2 and 12.2 g (n = 61). Seeds without pulp are 1.4–2.8 cm long, 0.9–1.7 cm wide, and 0.6–5.2 g in fresh weight (n = 191).
Fruit removal from the canopies
Fruits in the canopies were observed from June 2015 to August 2016. During this period, M. liukiuense had approximately three fruiting seasons: June–July 2015, February–April 2016, and May–August 2016. Seven fruiting branches from five trees were observed. Two types of time-lapse photograph recorders were used. One was for diurnal observations (Recolo IR7, Kingjim Co., Ltd., Tokyo, Japan), and the other was a camera with infrared LEDs for nocturnal observations (DVR-HC7310A, Hanwha Q CELLS Japan Co., Ltd. Tokyo, Japan). Observations started when the fruits were greenish-yellow and finished when all fruits moved away from the focal branches. Photographs were taken every 5–12 s according to the reaction speed of each memory card and battery cell.
The time-lapse photographs were considered continuous footage of when and what animals visited and what they did. The fate of each fruit was determined using the photographs. Behaviours of animals in the photographs were categorised as ‘visiting’, ‘eating’, ‘dropping’, and ‘carrying’. ‘Visiting’ indicated that animals appeared in photographs and handled no fruit, while ‘eating’ indicated biting fruits or ingesting pulp. Fruits were considered ‘dropped’ when successive photographs indicated the disappearance of fruits while the animals stayed. ‘Carrying’ indicated that animals with fruits in their mouths or bills disappeared from the photographs. When the fruits disappeared in successive photographs without any animals, it was considered ‘falling’, meaning that the fruits were dropped spontaneously.
Fruit removal from the ground
Fruits set on the ground under the fruiting crowns were observed for almost the entire period mentioned above. Observations were conducted in seven locations using the same type of cameras, as described above. A pair of cameras covered an area of approximately 2 m2 on the ground. The observations started when the ripe fruits began to fall spontaneously and ended when almost all the fruits disappeared from the adjacent fruiting trees. During the observations, about 10 ripe fruits collected from the focal trees were placed on the ground, and new ripe fruits were added when all fruit pulp was eaten up. The behaviours of visitors were categorised as ‘visiting, ‘eating’, ‘moving’, and ‘carrying’. ‘Visiting’ indicated that the animals handled no fruit, and ‘eating’ indicated biting fruits or ingesting pulp. ‘Moving’ indicated that animals moved the fruits, but the moved fruits could still be seen in the photographs. ‘Carrying’ indicated that animals with the fruits in their mouths or bills disappeared from the photographs.
Results
Fruit removal from the canopies
The total observation duration was 7340 h. Photography failed for 423 h (5.8% of the observation duration) due to rain, wind, or other mechanical problems.
The fates of 358 fruits in the canopies were determined (Table 1). Although 13% (n = 47) of fruits were missing or unidentified, 7.5% (n = 27) were carried away by animals, 48% (n = 171) were dropped by animals, and 32% (n = 113) fell spontaneously. An average of 82% ± 3.1% (SEM, n = 7 branches) were dropped under their mother trees.
Table 1. Fates of the fruits of Monoon liukiuense (Hatus.) B. Xue et R.M.K. Saunders during three fruiting seasons from June 2015 to August 2016 on Iriomote Island, Japan

* 1 Animals disappeared with the fruits.
* 2 Fruits remained in the photographs.
* 3 Seeds were left on the spot.
The most frequent visitor was the brown-eared bulbul (Hypsipetes amaurotis stejnegeri (Hartert, 1907)) (Table 2 and S1). The Yaeyama flying fox (Pteropus dasymallus yayeyamae Kuroda, 1933) was the most frequent animal dropping the fruits, followed by the brown-eared bulbul and large-billed crow (Corvus macrorhynchos osai (Ogawa, 1905)). These three volant animals were observed carrying away the fruits. The number of dispersed fruits per visit of the large-billed crow was 0.18, which was much higher than those of the Yaeyama flying fox (0.06) and brown-eared bulbul (0.01). The relative importance of dispersal for the large-billed crow and Yaeyama flying fox were comparable (48% and 44%, respectively), whereas that of the brown-eared bulbul was considerably less than the other two species.
Table 2. Animals that handled the fruits of Monoon liukiuense (Hatus.) B. Xue et R.M.K. Saunders during three fruiting seasons from June 2015 to August 2016 on Iriomote Island, Japan
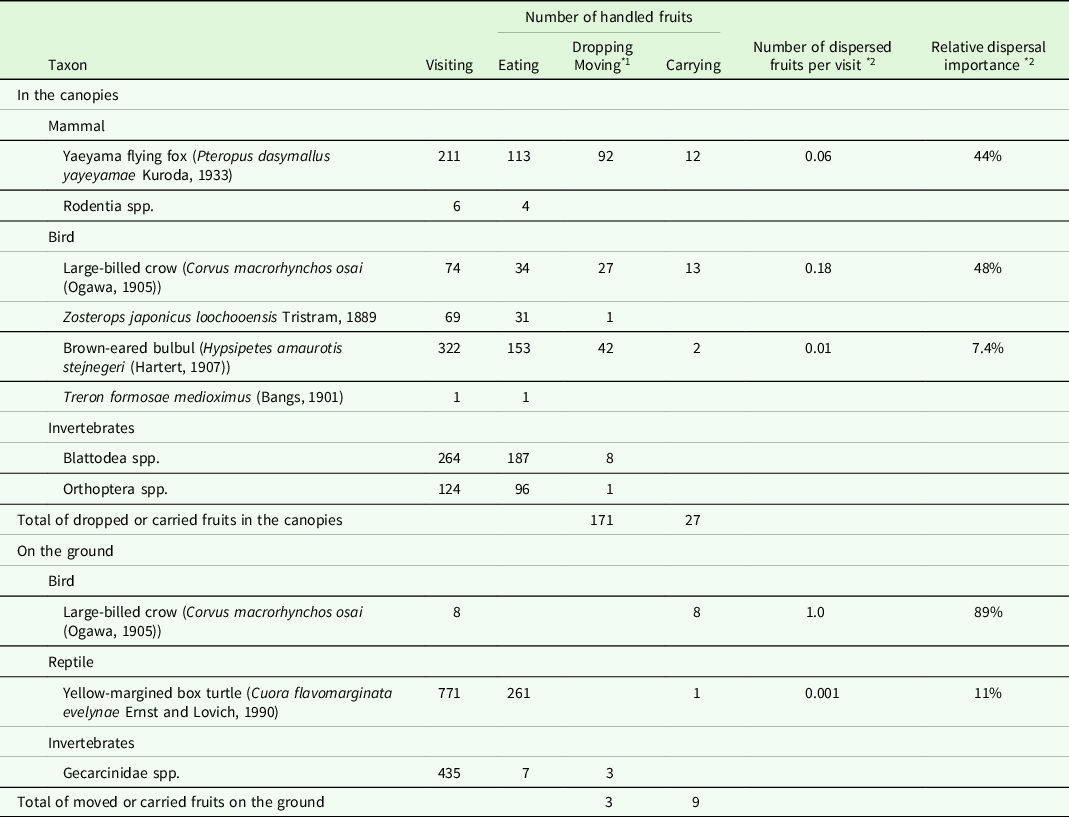
* 1 ‘Dropping’ in the canopies and ‘moving’ on the ground.
* 2 Calculated assuming that ‘carrying’ causes ‘dispersal’.
Fruit removal from the ground
The total observation duration was 5844 h. Photography failed for 333 h (5.7% of observation duration), mainly due to mechanical problems.
The fate of 222 fruits on the ground was determined (Table 1). Animals carried away 4.1% of the fruits (n = 9), whereas 1.4% (n = 3) were moved by animals but could still be seen in the photographs. An average of 90% ± 8.7% (SEM, n = 7 locations) were consumed in their original locations, and their seeds were left behind.
The most frequent epigeal visitor was the yellow-margined box turtle (Cuora flavomarginata evelynae Ernst and Lovich, 1990) (Table 2 and S2). The turtles consumed almost all the pulp of a fruit. One fruit was bitten and carried out of the camera’s view by a turtle when two individuals competed for it (Figure S2f). The large-billed crows were observed eight times and carried the fruits away on each occasion (Table 2 and S2). In this study, only these nine fruits were carried away from the ground.
Discussion
In the observations made in the canopies, an average of 82% of the fruits were dropped under the mother trees (Table 1). Howe & Vande Kerckhove (Reference Howe and Vande Kerckhove1981) observed fruit removal for wild nutmeg—whose seed size of similar to that of M. liukiuense—in Panama and revealed that an average of 38% of the fruits were either regurgitated by birds in the tree or were spontaneously dropped. The frequency of the fallen fruits in the present study (82%) was higher than for wild nutmeg. This result suggests that not all branches had sufficient primary seed dispersers.
On the ground, an average of 90% of the seeds were left in their original positions, while almost all pulp was consumed (Table 1). Several previous studies reported that terrestrial animals move most fruits from their original locations (e.g., Chauvet et al. Reference Chauvet, Feer and Forget2004; Dennis Reference Dennis2003; Forget Reference Forget1990, Reference Forget1992; Kitamura et al. Reference Kitamura, Suzuki, Yumoto, Poonswad, Chuailua, Plongmai, Noma, Maruhashi and Suckasam2004; Kitamura et al. Reference Kitamura, Suzuki, Yumoto, Poonswad, Chuailua, Plongmai, Maruhashi, Noma and Suckasam2006). The frequency of the seeds remaining in their original positions in the present study was much higher than in the previous studies above mentioned. This result suggests that Iriomote Island has almost no secondary seed dispersers for M. liukiuense.
Various organisms, including most of the large omnivores and carnivores reported on the island (Ohdachi et al. Reference Ohdachi, Ishibashi, Iwasa, Fukui and Saitoh2015; Takano Reference Takano1981), were observed both in the canopies and on the ground (Table S1 and S2). This suggested the exhaustive nature of my observations. Among the observed animals, the brown-eared bulbul, Yaeyama flying fox, yellow-margined box turtle, and large-billed crow carried the fruits of M. liukiuense.
Bulbuls (Pycnonotidae), such as the brown-eared bulbul, are the important avian dispersal agents for small (<14–15 mm) fruits in the Oriental region (Corlett Reference Corlett2017). However, the fruit of M. liukiuense may be too large (>16 mm) for the bulbul to fully swallow (Figure S2a, b). Although bulbuls picked some fruits with their bills from the branches, their visiting rates and the relative importance of dispersal were relatively low (Table 2). Thus, the brown-eared bulbul was not considered an effective seed disperser of M. liukiuense on Iriomote Island.
The Yaeyama flying fox could manipulate the fruit in both its mouth and foot (Figure S2c, d). The flying fox was considered a seed disperser of M. liukiuense, because some flying foxes held the fruits in their mouths and disappeared from the canopies. However, McConkey and Drake (Reference McConkey and Drake2006) reported that an insular flying fox in Tonga (Pteropus tonganus) lost its function as an effective seed dispersal agent at sites where its abundance fell below a certain threshold. The Yaeyama flying fox is an endangered species with an unknown population size (Kinjo & Nakamoto Reference Kinjo and Nakamoto2017). Therefore, it is uncertain whether the flying fox on Iriomote Island is abundant enough to play a significant role as a seed dispersal agent. However, the Yaeyama flying fox remains a potential seed disperser of M. liukiuense because of its ability to fly with the fruit.
On the ground, the yellow-margined box turtle carried the fruit in its mouth on one occasion when two turtles fought over a fruit (Figure S2f). However, the turtles seemed unable to swallow the seed because their heads were only slightly larger or almost similar in width to the fruits (Figure S2e). Therefore, this carrying event may have happened accidentally, and the turtles may not be a seed dispersal agent for M. liukiuense. However, Corlett (Reference Corlett2017) speculated that extinct Pleistocene giant tortoises (Testudinidae) of Wallacea had seed dispersal abilities. An extinct testudinid tortoise (estimated maximum carapace length of ca. 45 cm) was found in late Pleistocene deposits in the Ryukyu Islands (Takahashi et al. Reference Takahashi, Hirayama and Otsuka2018). Its carapace length was 2.4 times larger than that of the yellow-margined box turtle, which grows to 19 cm in adults (Ota et al. Reference Ota, Yasukawa, Fu and Chen2009). This extinct tortoise might have swallowed the whole fruits of M. liukiuense.
The large-billed crows were observed to carry away the fruits in their bills, both in the canopies and on the ground (Figure S2g, h). One of the crows was photographed carrying a fruit of Pandanus odoratissimus L. fil., which is >2 cm in diameter (Figure S2i). Because the fruit of M. liukiuense is smaller than that of P. odoratissimus, the crow could carry M. liukiuense fruits. The number of dispersed fruits per visit was the highest for the crow among all visitors, both in the canopies and on the ground (Table 2). Thus, the large-billed crow was considered the most effective seed disperser of M. liukiuense. However, some crows removed the seeds from the fruits and held only the seeds in their bills (Figure S2g). These behaviours suggested that on Iriomote Island, the crow was not only a potential seed disperser but also a predator of M. liukiuense seeds.
The results suggest that M. liukiuense on Iriomote Island has two potential seed dispersers, the Yaeyama flying fox and large-billed crow. Other close relatives of M. liukiuense, including Polyalthia spp., have been suggested to rely on seed dispersal by civets, hornbills, cassowaries, macaques, and flying foxes (Richards Reference Richards1990; Sengupta et al. Reference Sengupta, McConkey and Radhakrishna2014; Sethi & Howe Reference Sethi and Howe2012; Stocker & Irvine Reference Stocker and Irvine1983). These animals have not been reported on Iriomote Island, except for the Yaeyama flying fox (Ohdachi et al. Reference Ohdachi, Ishibashi, Iwasa, Fukui and Saitoh2015; Takano Reference Takano1981). While terrestrial rodents might play important roles as seed dispersers in Malaysian tropical rain forests (Yasuda et al. Reference Yasuda, Miura and Azman Hussein2000), no rodents were observed to carry any fruits in these observations (Table S1 and S2). Previous studies in intact forests reported that it was common for various frugivorous animals to disperse the fruit of any particular plant species (Gutier-Hion et al. Reference Gautier-Hion, Duplantier, Quris, Feer, Sound, Decoux, Dubost, Emmons, Erand, Hecketsweiler, Moungazi, Roussilhon and Thiollay1985; Kitamura et al. Reference Kitamura, Yumoto, Poonswad, Chuailua, Plongmai, Maruhashi and Noma2002). The seed disperser assemblage of M. liukiuense on Iriomote Island is not diverse and provides a simple dispersal process with no ground-dwelling agents. Combinations of multi-step dispersal processes provided by various agents are more beneficial for seed dispersal than most single-step processes (Vander Wall & Longland Reference Vander Wall and Longland2004). The seeds of M. liukiuense may not benefit sufficiently from the simple dispersal process observed in this study. Furthermore, the effectiveness of each potential disperser remains unclear, and both quantitative and qualitative data are required to clarify their roles (Schupp Reference Schupp1993; Schupp et al. Reference Schupp, Jordano and Gómez2010). In this study, only a few quantitative components, such as the visitation rates of frugivores and the number of fruits moved by them, were estimated. Therefore, further studies are required to reveal where the Yaeyama flying fox and large-billed crow carry the seeds, whether the seeds can survive and germinate there, and how the simple seed dispersal process affects the survival prospects of M. liukiuense. Additionally, it might be worthwhile collecting the seeds and establishing new populations in other localities to enhance the persistence of M. liukiuense.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467423000056
Acknowledgements
I thank Naoko Sashimura for sharing the initial ideas for this study. I acknowledge the Taketomi town government for granting permission to conduct this research. And, I also thank two anonymous reviewers for helpful comments on this manuscript.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.







