No CrossRef data available.
Article contents
Morpho-functional Analysis of the Head Glands in Three Auchenorrhyncha Species and Their Possible Biological Significance
Published online by Cambridge University Press: 12 September 2022
Abstract
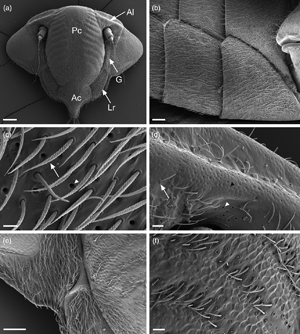
The Cicadomorpha Philaenus spumarius, Neophilaenus campestris, and Cicadella viridis are known transmitters of the bacterium Xylella fastidiosa. Here, we studied the ultrastructural organization of their cephalic glands. Our investigations with scanning, transmission, focused ion beam-scanning electron microscopes and light microscope revealed for the first time in Auchenorrhyncha the presence of two types of cephalic glands. Both belonged to the Class III epidermal glands, according to the Noirot and Quennedey classification. Type A glands were the most common, being mainly located around antennae, lorum, and gena. Moreover, these glands were observed also on the abdomen and thorax, always in association with sensilla trichoidea. The second type of glands (type B) were located exclusively at the apical part of the postclypeus in P. spumarius and N. campestris. The ultrastructural organization was similar in both types, being composed of a secretory cell and a conducting canal. Differences were observed in the width of the cuticular opening, being smaller in the type II glands. In addition, we have recorded the presence of a maxillary sensory pit in all species and described sensilla trichoidea ultrastructural organization. Finally, we discussed the ultrastructural organization of the glands and their potential biological role.
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America





