No CrossRef data available.
Article contents
Alumolukrahnite, CaCu2+Al(AsO4)2(OH)(H2O), the aluminium analogue of lukrahnite from the Jote mine, Copiapó Province, Chile
Published online by Cambridge University Press: 28 December 2022
Abstract
The new mineral alumolukrahnite (IMA2022–059), CaCu2+Al(AsO4)2(OH)(H2O), was found at the Jote mine, Copiapó Province, Chile, where it is a secondary alteration phase associated with conichalcite, coronadite, gypsum, olivenite, pharmacosiderite, rruffite and scorodite. Alumolukrahnite occurs as crude diamond-shaped tablets up to ~0.1 mm, intergrown in crude spherical aggregates. Crystals are apple green and transparent to translucent, with vitreous lustre and a white streak. The Mohs hardness is 3½. The mineral is brittle with irregular fracture and no cleavage. The calculated density is 4.094 g cm–3. Optically, alumolukrahnite is biaxial (+) with α = 1.73(1), β = 1.74(1) and γ = 1.76(1) (white light). The empirical formula, determined from electron microprobe analyses, is Ca1.01(Cu0.92Zn0.13)Σ1.05(Al0.96Fe0.01)Σ0.97(As0.985O4)2(OH)0.88(H2O)1.12. Alumolukrahnite is triclinic, P$\bar{1}$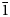 , a = 5.343(5), b = 5.501(5), c = 7.329(5) Å, α = 67.72(2), β = 69.06(2), γ = 69.42(2)°, V = 180.3(3) Å3 and Z = 1. Alumolukrahnite is a member of the tsumcorite group and is the Al analogue of lukrahnite.
, a = 5.343(5), b = 5.501(5), c = 7.329(5) Å, α = 67.72(2), β = 69.06(2), γ = 69.42(2)°, V = 180.3(3) Å3 and Z = 1. Alumolukrahnite is a member of the tsumcorite group and is the Al analogue of lukrahnite.
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Irina O Galuskina




