Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Graupner, T.
Götze, J.
Kempe, U.
and
Wolf, D.
2000.
CL for characterizing quartz and trapped fluid inclusions in mesothermal quartz veins: Muruntau Au ore deposit, Uzbekistan.
Mineralogical Magazine,
Vol. 64,
Issue. 6,
p.
1007.
Poolton, N R J
Smith, G M
Riedi, P C
Bulur, E
Bøtter-Jensen, L
Murray, A S
and
Adrian, M
2000.
Luminescence sensitivity changes in natural quartz induced by high temperature annealing: a high frequency EPR and OSL study.
Journal of Physics D: Applied Physics,
Vol. 33,
Issue. 8,
p.
1007.
Götze, J.
Plötze, M.
Tichomirowa, M.
Fuchs, H.
and
Pilot, J.
2001.
Aluminium in quartz as an indicator of the temperature of formation of agate.
Mineralogical Magazine,
Vol. 65,
Issue. 3,
p.
407.
Götze, J.
Tichomirowa, M.
Fuchs, H.
Pilot, J.
and
Sharp, Z.D.
2001.
Geochemistry of agates: a trace element and stable isotope study.
Chemical Geology,
Vol. 175,
Issue. 3-4,
p.
523.
Ioannou, S. E.
Götze, J.
Weiershäuser, L.
Zubowski, S. M.
and
Spooner, E. T. C.
2004.
Cathodoluminescence characteristics of Archean volcanogenic massive sulfide related quartz: Noranda, Ben Nevis and Matagami districts, Abitibi Subprovince, Canada.
Geochemistry, Geophysics, Geosystems,
Vol. 5,
Issue. 2,
Nasdala, Lutz
Götze, Jens
Hanchar, John M.
Gaft, Michael
and
Krbetschek, Matthias R.
2004.
Spectroscopic methods in mineralogy.
p.
43.
Witke, Klaus
Götze, Jens
Rößler, Ronny
Dietrich, Dagmar
and
Marx, Günter
2004.
Raman and cathodoluminescence spectroscopic investigations on Permian fossil wood from Chemnitz—a contribution to the study of the permineralisation process.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 60,
Issue. 12,
p.
2903.
Moxon, T.
and
Reed, S. J. B.
2006.
Agate and chalcedony from igneous and sedimentary hosts aged from 13 to 3480 Ma: a cathodoluminescence study.
Mineralogical Magazine,
Vol. 70,
Issue. 5,
p.
485.
Kibar, R.
Garcia-Guinea, J.
Çetin, A.
Selvi, S.
Karal, T.
and
Can, N.
2007.
Luminescent, optical and color properties of natural rose quartz.
Radiation Measurements,
Vol. 42,
Issue. 10,
p.
1610.
Babińska, Joanna
Dyrek, Krystyna
and
Wyszomirski, Piotr
2007.
EPR Study of Paramagnetic Defects in Clay Minerals.
Mineralogia,
Vol. 38,
Issue. 2,
p.
125.
Domanski, Marian
and
Webb, John
2007.
A Review of Heat Treatment Research.
Lithic Technology,
Vol. 32,
Issue. 2,
p.
153.
Götze, J.
and
Kempe, U.
2008.
A comparison of optical microscope- and scanning electron microscope-based cathodoluminescence (CL) imaging and spectroscopy applied to geosciences.
Mineralogical Magazine,
Vol. 72,
Issue. 4,
p.
909.
Götze, Jens
and
Kempe, Ulf
2009.
Cathodoluminescence and its Application in the Planetary Sciences.
p.
1.
Götte, J.
Möckel, R.
Kempe, U.
Kapitonov, I.
and
Vennemann, T.
2009.
Characteristics and origin of agates in sedimentary rocks from the Dryhead
area, Montana, USA.
Mineralogical Magazine,
Vol. 73,
Issue. 4,
p.
673.
Götze, J.
2009.
Chemistry, textures and physical properties of quartz — geological interpretation and technical application.
Mineralogical Magazine,
Vol. 73,
Issue. 4,
p.
645.
Sweeney, Ian J.
Chin, Karen
Hower, James C.
Budd, David A.
and
Wolfe, Douglas G.
2009.
Fossil wood from the middle Cretaceous Moreno Hill Formation: Unique expressions of wood mineralization and implications for the processes of wood preservation.
International Journal of Coal Geology,
Vol. 79,
Issue. 1-2,
p.
1.
Matysová, Petra
Rössler, Ronny
Götze, Jens
Leichmann, Jaromír
Forbes, Gordon
Taylor, Edith L.
Sakala, Jakub
and
Grygar, Tomáš
2010.
Alluvial and volcanic pathways to silicified plant stems (Upper Carboniferous–Triassic) and their taphonomic and palaeoenvironmental meaning.
Palaeogeography, Palaeoclimatology, Palaeoecology,
Vol. 292,
Issue. 1-2,
p.
127.
Graupner, Torsten
Niedermann, Samuel
Rhede, Dieter
Kempe, Ulf
Seltmann, Reimar
Williams, C. Terry
and
Klemd, Reiner
2010.
Multiple sources for mineralizing fluids in the Charmitan gold(-tungsten) mineralization (Uzbekistan).
Mineralium Deposita,
Vol. 45,
Issue. 7,
p.
667.
Hatipoğlu, Murat
Ajò, David
and
Sezai Kırıkoğlu, M.
2011.
Cathodoluminescence (CL) features of the Anatolian agates, hydrothermally deposited in different volcanic hosts from Turkey.
Journal of Luminescence,
Vol. 131,
Issue. 6,
p.
1131.
Paralı, L.
Garcia Guinea, J.
Kibar, R.
Cetin, A.
and
Can, N.
2011.
Luminescence behaviour and Raman characterization of dendritic agate in the Dereyalak village (Eskişehir), Turkey.
Journal of Luminescence,
Vol. 131,
Issue. 11,
p.
2317.
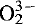 , E′1, [AlO4]0 [FeO4/M+]0 and [GeO4/M+]0. Centres of the type [TiO4/Li+]0 and [TiO4/H+]0, which were detected in quartz of the parent volcanics, are absent in agate. Generally, the abundance of
, E′1, [AlO4]0 [FeO4/M+]0 and [GeO4/M+]0. Centres of the type [TiO4/Li+]0 and [TiO4/H+]0, which were detected in quartz of the parent volcanics, are absent in agate. Generally, the abundance of 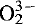 centres (silicon vacancy) and E′1 centres (oxygen vacancy) in agate is remarkably higher than in quartz. The high defect density in agates points to rapid growth of silica from a strongly supersaturated solution probably with a noncrystalline precursor.
centres (silicon vacancy) and E′1 centres (oxygen vacancy) in agate is remarkably higher than in quartz. The high defect density in agates points to rapid growth of silica from a strongly supersaturated solution probably with a noncrystalline precursor.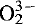 centres and E′1 centres. The emission intensity increases during electron bombardement due to the conversion of different precursors (e.g. ≡Si-O-H, ≡Si-O-Na groups) into hole centres. Another conspicuous feature in the CL spectra of agates is the existence of a yellow emission band centred at around 580 nm. The predominance of the yellow CL emission band and the high concentration of E′1 centres are typical for agates of acidic volcanics and are indicative of a close relationship between the two.
centres and E′1 centres. The emission intensity increases during electron bombardement due to the conversion of different precursors (e.g. ≡Si-O-H, ≡Si-O-Na groups) into hole centres. Another conspicuous feature in the CL spectra of agates is the existence of a yellow emission band centred at around 580 nm. The predominance of the yellow CL emission band and the high concentration of E′1 centres are typical for agates of acidic volcanics and are indicative of a close relationship between the two.



