Article contents
Millsite, CuTeO3·2H2O: a new polymorph of teineite from Gråurdfjellet, Oppdal, Norway
Published online by Cambridge University Press: 28 February 2018
Abstract
Millsite, CuTeO3·2H2O, is a new mineral from Gråurdfjellet in Oppdal, Norway. It occurs as a minor secondary phase alongside teineite, other copper secondaries and relict primary tellurides in a boulder of quartz-rich granite, which is probably a glacial erratic. Millsite is bright cyan to royal blue in colour. The mineral is transparent to slightly translucent with a vitreous lustre and has a perfect (100) cleavage. It is brittle, has a conchoidal fracture and a pale green streak. Millsite is optically biaxial (+), α = 1.756(5), β = 1.794(5), γ = 1.925calc and 2Vmeas = 60(1)°. Millsite has monoclinic space group P21/c, with a = 7.4049(2) Å, b = 7.7873(2) Å, c = 8.5217(2) Å, β = 110.203(3)°, V = 461.17(2) Å3 and Z = 4. The empirical formula is Cu0.99(Te0.98Se0.02)O3(H2O)2. The five strongest reflections in the powder X-ray diffraction pattern are [dhkl in Å (hkl, Irel%)]: 6.954 (100, 100), 3.558 (012, 64), 2.838 (12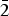 $\bar 2$, 47), 2.675 (211, 43) and 3.175 (210, 39). The crystal structure has been determined to R1 = 0.016, wR2 = 0.036 and GooF = 1.049. The diagnostic structural unit of millsite consists of a Cu2O6(H2O)4 dimer that is decorated with four TeO3 groups connecting adjacent dimers and defining (100) heteropolyhedral sheets. These heteropolyhedral sheets are only connected by layers of structurally significant hydrogen bonds and correlate with the (100) cleavage. Millsite is a polymorph of teineite with a unique configuration of the M2O6(H2O)4 dimer that leads to a sheet topology. No isostructural selenium or tellurium analogue exists. The monoclinic polymorph (P21/c) of chalcomenite ‘monoclinic-CuSeO3·2H2O’ hereafter, ahlfeldite and MgSeO3·2H2O have M2O6(H2O)4 dimers, but their configuration differs significantly from that of millsite and leads to a framework topology rather than a sheet. Teineite does not have a dimeric structure and so is fundamentally different from millsite. The sheet topology of millsite appears to be unique among tellurites.
$\bar 2$, 47), 2.675 (211, 43) and 3.175 (210, 39). The crystal structure has been determined to R1 = 0.016, wR2 = 0.036 and GooF = 1.049. The diagnostic structural unit of millsite consists of a Cu2O6(H2O)4 dimer that is decorated with four TeO3 groups connecting adjacent dimers and defining (100) heteropolyhedral sheets. These heteropolyhedral sheets are only connected by layers of structurally significant hydrogen bonds and correlate with the (100) cleavage. Millsite is a polymorph of teineite with a unique configuration of the M2O6(H2O)4 dimer that leads to a sheet topology. No isostructural selenium or tellurium analogue exists. The monoclinic polymorph (P21/c) of chalcomenite ‘monoclinic-CuSeO3·2H2O’ hereafter, ahlfeldite and MgSeO3·2H2O have M2O6(H2O)4 dimers, but their configuration differs significantly from that of millsite and leads to a framework topology rather than a sheet. Teineite does not have a dimeric structure and so is fundamentally different from millsite. The sheet topology of millsite appears to be unique among tellurites.
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Stuart Mills
References
- 5
- Cited by




