Article contents
Oberwolfachite, SrFe3+3(AsO4)(SO4)(OH)6, a new alunite-supergroup mineral from the Clara mine, Schwarzwald, Germany and Monterniers mine, Rhône, France
Published online by Cambridge University Press: 02 August 2021
Abstract
The new beudantite-group mineral oberwolfachite, ideally SrFe3+3(AsO4)(SO4)(OH)6, was discovered in two localities: Clara mine, Oberwolfach, Schwarzwald, Baden-Württemberg, Germany (holotype) and Monterniers mine, Lantignié, Rhône, Auvergne-Rhône-Alpes, France (cotype). The associated minerals are quartz, baryte, hematite, illite, goethite, beudantite and dussertite (Clara) and arsenogoyazite, jarosite, graulichite-(Ce), goethite and hematite (Monterniers). Oberwolfachite forms yellow to brown platy crystals up to 1 mm across or thick outer zones of mixed (oberwolfachite–beudantite) crystals up to 3 mm across. The lustre is adamantine and the streak is yellow. A distinct cleavage on {0001} is observed. Calculated density is 3.874 g⋅cm–3. The infrared spectra are given. The chemical composition of the holotype/cotype samples are (wt.%, b.d.l. = below detection limit): K2O 1.25/1.86, SrO 6.41/11.15, BaO 8.13/0.45, PbO 2.18/b.d.l., Al2O3 0.16/0.23, Fe2O3 38.99/39.98, La2O3 1.68/not determined, Ce2O3 1.28/2.06, P2O5 0.12/b.d.l., As2O5 17.55/16.55, SO3 12.86/14.99, H2O (calculated) 8.72/9.05, total 99.33/96.62. The empirical formula of the holotype sample is (Sr0.38Ba0.33K0.16Pb0.06La0.06Ce0.05)Σ1.04(Fe3+3.03Al0.02)Σ3.05[(SO4)1.00(AsO4)0.95(PO4)0.01](O6.16H6.00). The crystal structures of both samples were determined using single-crystal X-ray diffraction data and refined to R = 3.13% (holotype, 293 K) and 2.65% (cotype, 170 K). Oberwolfachite is trigonal, R${\bar 3}$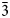 m, with a = 7.3270(3) Å, c = 17.0931(9) Å and V = 794.70(8) Å3 (holotype), and a = 7.298(2) Å, c = 16.908(3) Å and V = 779.8(4) Å3 (cotype); Z = 3. The strongest lines of the powder X-ray diffraction pattern of holotype [d, Å (I, %)(hkl)] are: 5.95 (56)(101), 3.664 (37)(110), 3.117 (16)(021), 3.082 (100)(113), 2.548 (15)(024), 2.280 (22)(107), 1.983 (26)(303) and 1.832 (19)(220).
m, with a = 7.3270(3) Å, c = 17.0931(9) Å and V = 794.70(8) Å3 (holotype), and a = 7.298(2) Å, c = 16.908(3) Å and V = 779.8(4) Å3 (cotype); Z = 3. The strongest lines of the powder X-ray diffraction pattern of holotype [d, Å (I, %)(hkl)] are: 5.95 (56)(101), 3.664 (37)(110), 3.117 (16)(021), 3.082 (100)(113), 2.548 (15)(024), 2.280 (22)(107), 1.983 (26)(303) and 1.832 (19)(220).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Koichi Momma
References
- 3
- Cited by




