Article contents
Electronic charge transfer properties of COF-5 solutions and films with intercalated metal ions
Published online by Cambridge University Press: 20 January 2020
Abstract
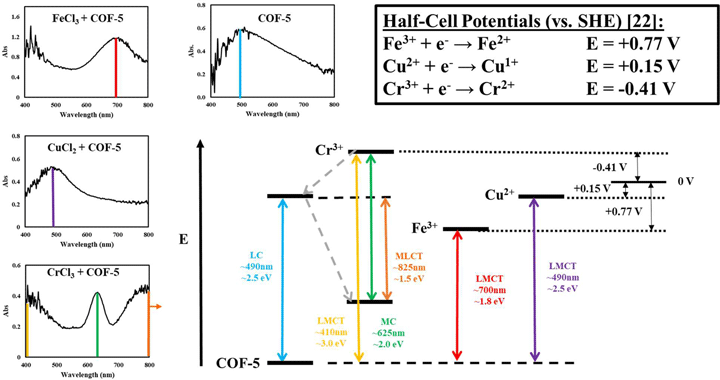
To investigate the manipulation of electromagnetic properties of two-dimensional materials, this effort characterizes charge transfer behavior of colloidal COF-5 (covalent organic framework) in the presence of various metal ions. A series of metal chloride compounds was introduced to COF-5 in solution and solid film phases and the interaction of the material with electromagnetic radiation was monitored across the visible region using electronic absorption spectroscopy. Notable changes were observed, quantified, and discussed for copper (II) chloride (CuCl2), chromium (III) chloride (CrCl3), and iron (III) chloride (FeCl3) with COF-5. Ligand-to-metal and metal-to-ligand charge transfer are explored as a possible mechanism for the observed electronic behaviors.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 1
- Cited by





