Article contents
Nickel-reduced graphene oxide composite foams for electrochemical oxidation processes: towards biomolecule sensing
Published online by Cambridge University Press: 13 July 2018
Abstract
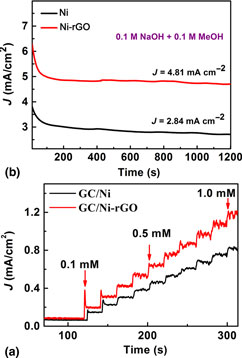
Metal–graphene composites are sought after for various applications. A hybrid light-weight foam of nickel (Ni) and reduced graphene oxide (rGO), called Ni-rGO, is reported here for small molecule oxidations and thereby their sensing. Methanol oxidation and non-enzymatic glucose sensing are attempted with the Ni-rGO foam via electrocatalytically, and an enhanced methanol oxidation current density of 4.81 mA/cm2 is achieved, which is ~1.7 times higher than that of bare Ni foam. In glucose oxidation, the Ni-rGO electrode shows a better sensitivity over bare Ni foam electrode where it could detect glucose linearly over a concentration range of 10 µM to 4.5 mM with a very low detection limit of 3.6 µM. This work demonstrates the synergistic effects of metal and graphene in oxidative processes, and also shows the feasibility of scalable metal–graphene composite inks development for small molecule printable sensors and fuel cell catalysts.
- Type
- 2D Nanomaterials for Healthcare and Lab-on-a-Chip Devices Prospective Articles
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
These authors contributed equally to this work.
References
- 5
- Cited by





