Article contents
Salt-templated platinum–palladium porous macrobeam synthesis
Published online by Cambridge University Press: 05 November 2018
Abstract
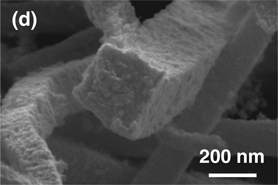
Here we present the synthesis of porous platinum–palladium macrobeams templated from high aspect ratio Magnus’ salt needle derivatives. The combination of [PtCl4]2− and/or [PdCl4]2− with [Pt(NH3)4]2+ ions results in salt needles ranging from 15 to 300 µm in length. Electrochemical reduction of the salt templates results in porous macrobeams with a square cross-section. Porous side wall texture and elemental composition was controlled with initial platinum to palladium salt ratio. Macrobeam free-standing films exhibited a specific capacitance up to 11.73 F/g and a solvent accessible surface area of 26.6 m2/g. These salt-templated porous platinum–palladium macrobeams offer a promising material for fuel cell catalysis.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
These authors contributed equally.
References
- 5
- Cited by




