Introduction
The jaguar Panthera onca is the largest felid in the Americas and is categorized on the IUCN Red List as Near Threatened (Caso et al., Reference Caso, Lopez-Gonzalez, Payan, Eizirik, de Oliveira and Leite-Pitman2008). Although the jaguar's distribution has been reduced by more than 50% within the past century (Seymour, Reference Seymour1989; Sanderson et al., Reference Sanderson, Redford, Chetkiewicz, Medellín, Rabinowitz, Robinson and Taber2002), it still ranges from New Mexico and Arizona in the USA to the north of Argentina, occurring in a variety of environments but with the remaining populations facing varying prospects of long-term survival.
Top predators such as the jaguar play an important role in the ecosystems in which they occur (Terborgh et al., Reference Terborgh, Estes, Paquet, Ralls, Boyd-Heger, Miller, Noss, Soulé and Terborgh1999), limiting the number of herbivores and thereby reducing the pressure they exert on plants (Terborgh, Reference Terborgh1988; Miller et al., Reference Miller, Dugelby, Foreman, Martinez del Rio, Noss and Phillips2001). This top-down regulation by predators maintains diversity, and their removal reduces species richness and increases populations of some species of small- and medium-sized carnivores and omnivores (Fonseca & Robinson, Reference Fonseca and Robinson1990; Terborgh et al., Reference Terborgh, Lopez, Tello, Yu, Bruni, Laurance and Bierregaard1997; Miller et al., Reference Miller, Dugelby, Foreman, Martinez del Rio, Noss and Phillips2001; Ripple & Beschta, Reference Ripple and Beschta2006). Jaguars are sensitive to human disturbance and require large tracts of habitat (Weber & Rabinowitz, Reference Weber and Rabinowitz1996). This may explain why, although widely distributed in Brazil, viable jaguar populations are mostly restricted to large protected areas (Silveira & Jácomo, Reference Silveira, Jácomo, Medellín, Equihua, Chetkiewitcz, Crawshaw, Rabinowitz and Redford2002).
Although estimation of density is a basic requirement for assessing the status of a population, jaguar densities have been little studied in Brazil. The 36 parks and other protected areas in the 800,000 km2 caatinga, the country's third largest biome, comprise 7.1% of the total area but only 1.21% of the area is under integral protection (Capobianco, Reference Capobianco, Camargo, Capobianco and Oliveira2002). The environmental conditions of this biome (high temperatures, poor soil fertility and a short rainy season) do not favour large-scale agriculture. Nevertheless, an estimated 30% of the caatinga has been altered by man, especially for agriculture (MMA, Reference de Araújo, Rodal and Barbosa2005). However, hunting of native wildlife appears to be a major threat to many game species and top predators inhabiting this biome (Leat et al., Reference Leal, Da Silva, Tabarelli and Lacher2005).
We used a camera-trap survey to identify jaguars and used mark-recapture models (Karanth & Nichols, Reference Karanth and Nichols1998) to obtain the first estimate of jaguar density in one of the caatinga's most important National Parks.
Study area
The 129,140 ha Serra da Capivara National Park is in the south of the state of Piauí, north-east Brazil (Fig. 1). Temperatures are 12–45°C, the rainy season is from October to April (Emperaire, Reference Emperaire1984), and mean total annual precipitation is 644 mm (SMAPR, 1994). Eight habitat types have been described for the Park but a 6- to 10-m-tall shrubby vegetation predominates (Emperaire, Reference Emperaire1984). Altitude is 280–600 m and the topography consists of a main plateau bounded by 50- to 200-m cliffs and dissected by valleys and canyons. There is no natural permanent water within the Park but a system of artificial waterholes has been constructed in the past 15 years. The area surveyed comprises the southern and central portions of the Park and consists primarily of high shrubby caatinga vegetation with patches of low caatinga and dense shrubby to arboreal caatinga vegetation (Fig. 1).
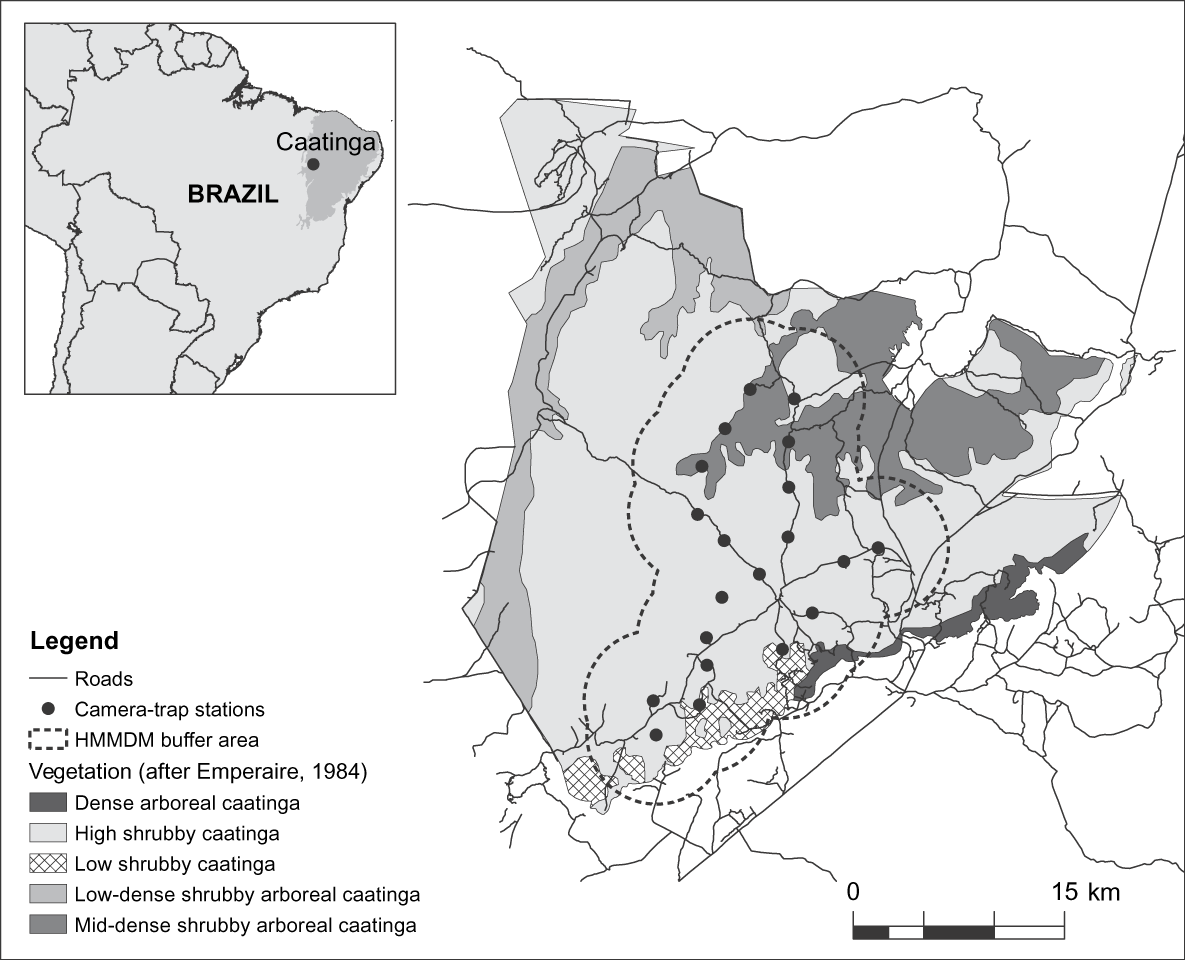
Fig. 1 Location of camera-trap stations and the half of the mean maximum distance moved buffer (HMMDM; see text for details) in the Serra da Capivara National Park, with the major vegetation types. The inset indicates the location of the Park in the caatinga, north-east Brazil.
Methods
The pattern of spots on a jaguar's coat allows the identification of individuals in photographs (Wallace et al., Reference Wallace, Gomez, Ayala and Espinoza2003; Maffei et al., Reference Maffei, Cuéllar and Noss2004; Silver, Reference Silver2004; Silver et al., Reference Silver, Ostro, Marsh, Maffei, Noss and Kelly2004; Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006). Photographs also allow the identification of gender (Silver et al., Reference Silver, Ostro, Marsh, Maffei, Noss and Kelly2004). From August to October 2007 we set 20 survey stations, each consisting of two cameras facing each other so as to obtain simultaneous photographs of both sides of any passing jaguars. Camera stations were placed along Park roads, each station a maximum of 3.4 km from the nearest other station (mean distance = 2.9 ± SD 0.4 km; Fig. 1), ensuring that within the sampled area camera trap coverage left no gaps greater than 10 km2. This value is based on the smallest home range recorded for jaguars and is used to attempt to ensure that all individuals are potentially exposed to camera traps (Silver, Reference Silver2004; Silver et al., Reference Silver, Ostro, Marsh, Maffei, Noss and Kelly2004). We used passive-sensor Camtrakker (CamTrack South Inc., Watkinsville, USA) camera traps model Original 35 mm, activated by heat and motion. Cameras were set to photograph during day and night, with a 5-minute delay between photos. They were checked at 15-day intervals for replacement of film and batteries. As we used two cameras per station and checked them regularly, sampling gaps were rare. However, where gaps occurred because of a malfunction or because film or battery finished, the respective number of days was not considered in the calculation of effort.
Jaguar abundance was estimated using the mark-recapture models implemented in CAPTURE (Otis et al., Reference Otis, Burnham, White and Anderson1978; Rexstad & Burnham, Reference Rexstad and Burnham1991). This method assumes that individuals can be identified to determine whether they have been captured and recaptured. To obtain individual capture histories we identified jaguars from their unique spot patterns and divided the trapping period into 14 trapping sessions of 6 days each, noting when each identified individual was captured (Table 1). The duration of 6 days minimized sessions with zero captures to increase capture probability, thus meeting the recommendations of Otis et al. (Reference Otis, Burnham, White and Anderson1978) of a minimum capture probability of 0.1 while simultaneously maximizing the number of recaptures.
Table 1 Capture histories of the 12 jaguars Panthera onca identified in the Serra da Capivara National Park (Fig. 1) in 2007, during a camera-trapping study (individual 4 was a cub and is not included here or in the density estimates). An entry of 1 indicates capture of the individual on the respective 6-day trapping occasion.

CAPTURE includes a series of closed population models, which assume that during the study period no recruitment (birth or immigration) or loss (deaths or emigrations) of individuals occurs (White et al., Reference White, Anderson, Burnham and Otis1982; Wilson & Anderson, Reference Wilson and Anderson1985). A test for population closure is implemented in CAPTURE. For jaguars a maximum sampling period of 2–3 months is recommended to meet this assumption (Silver, Reference Silver2004). We used 2.5 months. Considering that jaguars have a slow reproductive cycle and are territorial (Seymour, Reference Seymour1989), we believe that this duration is a reasonable approximation of population closure.
The models in CAPTURE consider various sources of variation in capture probability for an individual in a single trapping occasion: time, a behavioural response to trapping (i.e. differences in first capture and recapture), individual heterogeneity, and combinations of these sources. The software has a discriminate function procedure that selects the most appropriate model for the data based on a number of goodness-of-fit and between model tests.
To assess jaguar density the estimated abundance was divided by the effective sampled area, which contains the area defined by the camera traps and a buffer around this polygon. The objective of this buffer was to include individuals whose home ranges are only partially contained within the sampled area (Silver, Reference Silver2004). Buffer width was calculated as half of the mean maximum distance moved (HMMDM) between multiple captures of individuals during the survey period (Wilson & Anderson, Reference Wilson and Anderson1985). Soisalo & Cavalcanti (Reference Soisalo and Cavalcanti2006) suggested that using HMMDM potentially overestimates jaguar densities and that using the full mean maximum distance moved (MMDM) may be more realistic. There is no consensus on which distance to use and both are ad hoc approaches made necessary by the absence of independent movement data. As most jaguar studies use HMMDM we focus our interpretation, for comparative purposes, on HMMDM-based results. However, we also present density estimates using MMDM for buffer calculation.
Results
Between August and October 2007 we accumulated a sampling effort of 1,249 camera-trap nights. A total of 77 jaguar photographs were obtained and 12 different adult individuals were identified (Table 1). Capture frequencies were 1–17 times and the gender of 10 individuals could be determined (four females and six males), i.e. a female:male ratio of 1:1.4. Melanic jaguars comprised 33% of individuals identified (n = 4). Melanic jaguars were identified individually by scars and by their spot pattern, which can be observed if the individual is sufficiently close to the camera trap that the flashlight reveals the rosettes against the dark background coat colour.
CAPTURE results suggested a closed population (z = 0.123, P = 0.549) and recommended the model Mb as the best population estimator. CAPTURE calculated a capture probability (p) of 0.118 and a recapture probability (c) of 0.426. The abundance estimate (N) was 14 ± SE 3.643, with a confidence interval of 13–33. For the buffer calculation eight jaguars captured more than once were considered. These individuals were registered by at least two camera-trap stations and their HMMDM was 4.95 ± SD 1.93 km2. Based on this buffer the effective sampled area was 524 ± SD 157 km2, which resulted in a density estimate of 2.67 ± SE 1.06 individuals per 100 km2. For comparison, using the MMDM of 9.90 ± SD 3.87 km, the effective sampled area was 1,100 ± SD 455 km2 and jaguar density was estimated at 1.28 ± SE 0.62 per 100 km2.
Discussion
Although CAPTURE selected the behavioural model Mb as the best population estimator, with probability of recapture being higher than that of initial capture, we do not think there was a ‘trap-happy’ response to our cameras as there was no bait or lure associated with the traps. Camera-trapping jaguars in the Brazilian Pantanal, Soisalo & Cavalcanti (Reference Soisalo and Cavalcanti2006) also found model Mb to be the most appropriate and attributed this to the fact that their camera traps were set in places regularly used by jaguars. Similarly, the high recapture rates we observed probably resulted from our camera traps being set along roads. As reliability of model choice in CAPTURE can be weak (Stanley & Burnham, Reference Stanley and Burnham1998), we believe that choice of model Mb is probably an artefact of the small sample size rather than an indication of a behavioural response by the animals to trapping. In territorial mammals individual heterogeneity in capture probability is likely to occur (Karanth & Nichols, Reference Karanth and Nichols1998). As Mb tends to underestimate population size if other sources of variation in capture probability are present (Otis et al., Reference Otis, Burnham, White and Anderson1978) we consider our abundance estimate conservative.
The abundance of prey species is a determining factor for the abundance of large predators (Schaller, Reference Schaller1972; Karanth & Nichols, Reference Karanth and Nichols1998; Karanth et al., Reference Karanth, Nichols, Samba Kumar, Link and Hines2004), with medium- to large-sized mammals being the preferred prey of jaguars (López González & Miller, Reference López González and Miller2002). In the Pantanal high prey availability may be responsible for the high jaguar densities of 6.7 individuals 100 km-2 (Soisalo & Cavalcanti, Reference Soisalo and Cavalcanti2006) compared to other areas in Brazil (Table 2). Semi-arid systems such as caatinga are characterized by a low mean annual precipitation that results in low plant productivity (Davidson, Reference Davidson1977) and herbivore abundance (Chase et al., Reference Chase, Downing and Shurin2000). Medium- to large-sized jaguar prey species in the Orders Perissodactyla and Artiodactyla, such as tapirs Tapirus terrestris, deer Mazama spp. and peccaries Tayassu spp., are scarce in the caatinga, probably because of the low productivity and recent hunting pressure. These species are now only relict populations (Oliveira et al., Reference Oliveira, Gonçalves, Bonvicino, Leal, Tabarelli and Silva2003). In addition, the caatinga is characterized by small-scale subsistence farming with low agricultural production (MMA, 2007). The rural population is poor and poaching is common (Leal et al., Reference Leal, Da Silva, Tabarelli and Lacher2005), providing access both to a protein source and to money from selling excess game (Graffin, Reference Graffin2007). Depletion of the prey base may thus be potentially limiting the jaguar population.
Table 2 Jaguar densities in Brazil estimated using camera-trap data in combination with mark-recapture models.
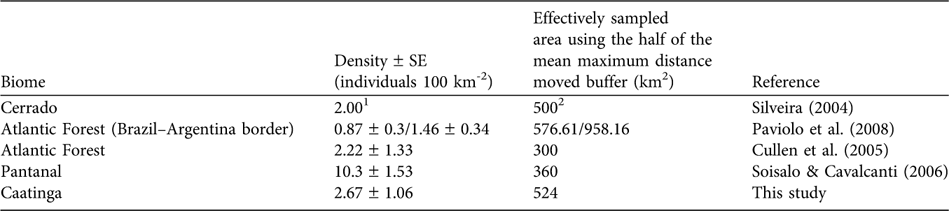
1 No SE was calculated for density
2 Estimated using a mean home range-based buffer
Previous studies, based on relative abundance indexes (Wolff, Reference Wolff2001), have suggested that densities of jaguars and of medium- to large-sized prey species such as deer, peccaries and giant anteaters Myrmecophaga tridactyla are low in the Serra da Capivara National Park (SMAPR, 1994; Wolff, Reference Wolff2001). Our estimate of jaguar density is thus higher than we expected. Our findings could indicate an adaptability of jaguars to feed on more readily available, smaller prey species in the caatinga. The small armadillos (Dasypus sp. and others) are part of the jaguar's diet in the Park (Olmos, Reference Olmos1993), and have also been reported to constitute part of the species’ diet in the Atlantic Forest of Brazil (Garla et al., Reference Garla, Setz and Gobbi2001). Since 2000 a strong and effective patrolling system has been implemented in the Park, with increased control of poaching. This may have helped the recovery of some populations of medium- to large-sized prey species. The park-wide system of artificial waterholes may also be benefiting these species. Additional studies of jaguar diet and prey availability, which we are currently conducting, will help to clarify this situation.
As our effective sampled area of 524 km2 covered nearly half of the Serra da Capivara National Park and was primarily composed of high shrubby caatinga, the predominant vegetation type (Fig. 1), we extrapolated our estimate of jaguar density to the entire Park. This extrapolation indicates that the Park may hold up to 34 adult jaguars. These results suggest that some areas in the caatinga still have the potential to sustain important jaguar populations. However, the situation in this well-protected Park probably does not reflect the reality for the other, mostly unprotected, areas of this biome: medium- to large-sized prey species, which generally comprise the bulk of the jaguar's diet (López González & Miller, Reference López González and Miller2002), are naturally sparse in this semi-arid environment (Oliveira et al., Reference Oliveira, Gonçalves, Bonvicino, Leal, Tabarelli and Silva2003) and their populations are further depleted by poaching (Leal et al., Reference Leal, Da Silva, Tabarelli and Lacher2005). In addition, jaguar habitat is extremely fragmented (Castelletti et al., Reference Castelletti, Silva, Tabarelli, Santos, Silva, Tabarelli, Fonseca and Lins2004) and there is a lack of information on the distribution, ecology and status of the caatinga jaguar (Oliveira, Reference Oliveira, Medellín, Equihua, Chetkiewitcz, Crawshaw, Rabinowitz and Redford2002). The work presented here is part of an ongoing study of these topics in Serra da Capivara National Park and the nearby Serra das Confusões National Park. Our results have been provided to the Park authorities for consideration in park management plans. However, similar studies in additional areas are also needed for an improved understanding of the status of the jaguar and to provide data for conservation plans for this species in the caatinga.
Acknowledgements
We appreciate the support provided by the Fundação Museu do Homem Americano and the park administration of Instituto Brasileiro do Meio Ambiente, which made this study possible. The study was funded by the Jaguar Conservation Fund (JCF), Oregon Zoo's Future for Wildlife Grants Program, University of Brasília, Idea Wild, the Memphis Zoo and the Woodland Park Zoo. We thank Barbara Zimbres, Debora Schistek, Eliot Cohen and Marina Carvalho for field assistance. A scholarship grant to SA was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and for RS by the German Academic Exchange Service. This study is part of the JCF jaguar population monitoring programme in Brazil. JM-F also received financial support from CNPq.
Biographical sketches
Leandro Silveira is interested in large-scale predator conservation, especially of the jaguar, and also researches methods for reducing predator/rancher conflict. Anah T.A. Jácomo focuses on large mammal conservation and management in human-dominated landscapes. Samuel Astete studies jaguar ecology in the caatinga to improve the knowledge of this and other species in this little studied biome. Rahel Sollmann focuses on jaguar ecology and conservation status in the fragmented landscape of the central Brazilian cerrado. Natália M. Tôrres uses ecological niche modelling to evaluate the jaguar's distribution under current and future climate scenarios to identify priority areas for jaguar conservation. Mariana M. Furtado is investigating the epidemiological relationship between jaguars and domestic animals in Brazil to understand the role diseases play in jaguar conservation. Jader Marinho-Filho focuses his work on mammal zoology, ecology and conservation.







