Article contents
Haplotype comparisons of Echinococcus granulosus sensu lato via mitochondrial gene sequences (co1, cytb, nadh1) among Pakistan and its neighbouring countries
Published online by Cambridge University Press: 26 April 2021
Abstract
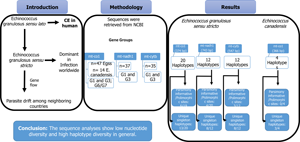
Echinococcus granulosus sensu lato (s.l.) is a zoonotic parasite that causes cystic echinococcosis (CE) in humans. However, E. granulosus sensu stricto (s.s.) is considered the predominant species in CE infections worldwide. According to the population genetic diversity and structure of E. granulosus s.l., gene flow can explain the parasite drift among the neighbouring countries of Pakistan. The mitochondrial (mt) co1 (n = 47), nadh1 (n = 37) and cytb (n = 35) nucleotide sequences of E. granulosus s.l. isolates from Pakistan, Iran, China and India were retrieved from the National Centre for Biotechnology Information database to determine the genealogical relationships. The sequences were grouped as the mt-co1 (genotypes G1 and G3, G6-G7), mt-cytb (genotypes G1 and G3), and mt-nadh1(genotypes G1 and G3). The data were analysed using bioinformatic tools. A total of 19 polymorphic sites for the mt-co1 sequence (374 bp) were observed of which 31.6% (6/19) were parsimony-informative sites. Unique singleton haplotypes within the E. granulosus s.s. haplotype network based on the mt-co1 gene were highly prevalent (68.4%; 13/19) in Pakistani isolates followed by Chinese, Indian and Iranian isolates; four polymorphic sites were detected in the E. canadensis (G6/G7). In E. canadensis mt-co1 haplotype network, 75% (3/4) unique singleton haplotypes were from the Iranian isolates. Twelve polymorphic sites were found using the mt-cytb sequence (547 bp); 25% (3/12) were parsimony-informative and there were 66.7% (8/12) unique singleton haplotypes within the mt-cytb haplotype network in E. granulosus s.s. with the most reported from Pakistan followed by Iran and China. 20 polymorphic sites were detected in E. granulosus s.s. mt-nadh1 sequences (743 bp); 20% (4/20) were parsimony-informative. There were 66.7% (8/12) main single haplotypes within the mt-nadh1 haplotype network, with the most reported from Pakistan followed by that from India, Iran and China. The sequence analyses show low nucleotide diversity and high haplotype diversity in general.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 2
- Cited by





