Introduction
The projected change in climate for the Great Lakes Region will affect agricultural production in many ways. Since 1951, the Great Lakes Region has seen an increase in annual average air temperature of 1.3 C, 14% increase in total precipitation, and a 35% increase in heavy precipitation events (GLISAP 2014). Additionally, the Great Lakes Integrated Sciences and Assessments Program projects yearly precipitation will increase; however, this precipitation will fall in heavy rainfall events, leaving more days per year that receive little or no precipitation.
Given these projections, drought events may become more common in the Great Lakes Region. Water stress is a critical abiotic stress during corn (Zea mays L.) development (Witt et al. Reference Witt, Galicia, Lisec, Cairns, Tiessen, Araus, Palacios-Rojas and Fernie2012). Although corn is a C4 plant, able to photosynthesize when the stomates are closed (Lopes et al. Reference Lopes, Araus, Van Heerden and Foyer2011), drought conditions can significantly impact plant growth and phenology (Hatfield and Prueger Reference Hatfield and Prueger2015). Water stress during the vegetative growth stages reduces stem and leaf cell expansion, resulting in shorter plants with less leaf area (Licht and Archontoulis Reference Licht and Archontoulis2017). Additionally, during the reproductive growth stages, substantial yield loss can occur if water stress occurs during silking and grain fill (Claassen and Shaw Reference Claassen and Shaw1970).
In conjunction with water stress, high temperatures can significantly impact corn yield, specifically during the pollination phase (Hatfield and Prueger Reference Hatfield and Prueger2015). Corn pollen viability decreased by 59% when exposed to temperatures above 32 C compared with 27 C (Herrero and Johnson Reference Herrero and Johnson1980). Further, extreme temperatures can impact the rate of maturation and senescence. Hatfield and Prueger (Reference Hatfield and Prueger2015) reported that when corn plants were grown under the extreme temperature of 34 C for 120 d, total vegetative biomass increased by 20% compared with total vegetative biomass of plants grown under 30 C conditions; however, yield decreased by 88%.
Since the intensification of modern-day production agriculture began, drought-tolerant corn hybrids have not been commercially available to protect against water-deficiency stress caused by drought events. Recently, an emphasis toward genetic modification of crops, specifically corn, has led to an increase in research and development in many agricultural companies and universities (Bruns Reference Bruns2019). DroughtGard™ corn was developed by Bayer Crop Science through the constitutive expression of cold shock protein B (cspB) from Bacillus subtilis to improve performance of corn under drought conditions (Wang et al. Reference Wang, Burzio, Koch, Silvanovich and Bell2015). cspBs from the bacterial species B. subtilis bind to single-stranded nucleic acids, therefore acting as RNA chaperones (Zeeb and Balbach Reference Zeeb and Balbach2003). Functionally, cspB enables corn plants to decrease the rate of water absorption from the soil in dry conditions, enabling the corn plant to withstand drought conditions for longer periods of time than conventional non–drought tolerant hybrids (Eisenstein Reference Eisenstein2013).
Drought-tolerant corn hybrids are often marketed as providing drought and heat tolerance in low-yielding environments where water is scarce (Newell et al. Reference Newell, Roozeboom, Kluitenberg and Ciampitti2015). Currently, limited research has been conducted on drought-tolerant corn hybrids, and results from these studies have shown no difference in water-use efficiency and grain yield between the DroughtGard™ corn hybrid and a non–drought tolerant hybrid (Bruns Reference Bruns2019; Kisekka et al. Reference Kisekka, Lamm and Holman2015).
In addition to the abiotic stressors of moisture and temperature, a significant biotic stress is weed competition. Weeds are troublesome, aggressive, and competitive plants that share a similar trophic level with crops, allowing them to compete for scarce resources of water, light, and nutrients (Ramesh et al. Reference Ramesh, Matloob, Aslam, Florentine and Chauhan2017). With the projected increase in erratic rainfall events, weed impacts on agricultural commodities are expected to change, resulting in shifts in competition (Ramesh et al. Reference Ramesh, Matloob, Aslam, Florentine and Chauhan2017). Rodenburg et al. (Reference Rodenburg, Riches and Kayeke2010) hypothesizes that during extended drought conditions and increased temperatures, C4 weeds will outcompete C3 weeds. Reasons for this shift surround photosynthetic efficiency; C4 weeds have a higher water-use efficiency than C3 weeds, thus C4 weeds may become more dominant under dry environmental conditions (Singh et al. Reference Singh, Singh and Singh2011; Ward et al. Reference Ward, Tissue, Thomas and Strain1999).
Common lambsquarters (Chenopodium album L.) is a C3 broadleaf summer annual weed that is common in many agricultural fields in the Great Lakes Region (Korres et al. Reference Korres, Norsworthy, Tehranchian, Gitsopoulos, Loka, Oosterhuis, Gealy, Moss, Burgos and Miller2016). Without crop competition, C. album is a prolific seed producer and can produce 75,600 to 150,400 seeds plant−1; however, in competition with corn, C. album seed production is reduced to 110 to 3,600 seeds plant−1 (Colquhoun et al. Reference Colquhoun, Boerboom, Binning, Stoltenberg and Norman2001). Furthermore, in a list of the world’s worst weeds by Holm et al. (Reference Holm, Plucknett, Pancho and Herberger1977), C. album was in the top 10. In competition with corn, C. album has been found to decrease yield by 12% with only 4.9 plants 1 m−1 of row (Beckett et al. Reference Beckett, Stoller and Wax1988).
Like crops, weeds are also subject to drought conditions (Maganti et al. Reference Maganti, Weaver and Downs2005). Chenopodium album height was reduced by 29% to 55% under extreme drought conditions (no water for 21 d after initial establishment period) compared with continuously watered plants (Maganti et al. Reference Maganti, Weaver and Downs2005). Furthermore, in the same study, C. album shoot dry weight was reduced by 28% to 64% under drought conditions compared with the non-drought treatment.
As outlined earlier, future climate scenarios for the Great Lakes Region predict more precipitation in heavy rainfall events, leaving more days during the growing season that have little or no precipitation, polarizing the wet and dry periods. Given this, it is important that producers modify integrated weed management plans to help mitigate potential negative impacts on crop growth, weed competition, and yield. The introduction of drought-tolerant corn hybrids into the market raises the question of how these new hybrids will perform under a combination of drought conditions and weed pressures in the Great Lakes Region. Therefore, the objective of this research was to evaluate growth and physiology of the drought-tolerant corn hybrid and C. album under two watering regimes and nine competition scenarios.
Materials and Methods
Greenhouse experiments were conducted February to April 2020 at Michigan State University in East Lansing, MI, USA. The study followed a completely randomized block design with four replications, and two experimental runs were performed. Treatments included factorial combinations of two levels of water stress that simulate no and moderate stress (Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2016), 100% and 50% soil volumetric water content (VWC), and nine corn:C. album densities: 1:0, 1:2, 1:4, 1:6, 1:9, 0:2, 0:4, 0:6, and 0:9.
Chenopodium album seed was collected from the Michigan State University Beef Farm in 2018, threshed, and stored at 5 C until experiments began. To break dormancy and ensure uniform germination, seeds were treated with a cold-water bath for 7 d before the experiment. The corn hybrid used in this study was DKC47-27 DroughtGard Double Pro® (Bayer Crop Science, Whippany, NJ, USA). Soil used for the study was a 50:50 mixture of greenhouse media (Suremix, Michigan Grower Products, Inc.™ Galesburg, MI, USA) and sterilized field soil. Field soil was screened with a 6-mm sieve for uniform consistency. The final soil mixture was a sandy loam with a pH of 7.4, 15.4% organic matter, 64.9% sand, 9.6% silt, and 10.1% clay.
Soil water-holding capacity was determined following the methods of Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2016). Final water-holding capacity was calculated using Equation 1:
where WC is the total water content, W w is the weight of the saturated pot of soil, W d is the weight of the dry pot of soil, and d is the density of water (Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2016). Greenhouse temperature was set to 27 C (diurnal range 25 to 29 C) with a 16-h photoperiod.
To ensure both C. album and corn plants emerged at the same time, C. album seeds were planted 4 d before corn into 20-cm-wide and 14-cm-deep (2.8-L) pots. Chenopodium album seedlings were thinned to desired densities once plants reached the cotyledon stage. To ensure uniform germination, pots were watered to 100% soil VWC until corn reached the 2-leaf stage and C. album reached 2 cm. VWC was monitored daily using a Field Scout TDR 300 Meter (Spectrum Technologies, Aurora, IL, USA) and then watered accordingly to achieve desired moisture levels. In both experimental runs, corn height and growth stage and weed height and leaf number were measured every 7 d. Corn photosynthetic efficiency was measured every 14 d in both experimental runs. Photosynthesis readings (Phi2, quantum yield of photosynthesis II; PhiNO, quantum yield of other unregulated losses; and PhiNPQ, quantum yield of non-photochemical quenching) were taken using a MultispeQ V 2.0 (PhotosynQ, East Lansing, MI, USA). Aboveground biomass of corn and weeds was harvested at 3 wk (Run 1, cut 1-wk short due to onset of COVID restrictions) and 4 wk (Run 2) after water stress began, dried at 60 C, and weighed.
Statistical Analyses
Data from experimental runs were combined after examining side-by-side box plots of the residuals, as well as a Levene’s test for unequal variances. Normality assumptions were assessed by examining normal probability plots of the residuals and histograms. Growth parameters of corn and C. album were analyzed via nonlinear regression using the drc package in R (R Core Team 2021) following the methods outlined in Knezevic et al. (Reference Knezevic, Streibig and Ritz2007). Three-parameter log-logistic models were fit to weed height and leaf number (Equation 2) and three-parameter Weibull models were fit to corn growth stage and height (Equation 3). Model fit was evaluated using the drc modelFit function in R, which is a lack-of-fit test, only models with P-values > 0.05 were chosen for analysis (Table 1).
Table 1. List of models used for greenhouse growth parameters. a

a Models were chosen using the modelFit function in R (R Core Team 2021).
For all equations, Y is the response variable (height, leaf number, or growth stage); x is the number of days after water-stress treatment initiation; c and d are the lower and upper limits, respectively; b is the relative slope around e; and e is the inflection point (Streibig Reference Streibig1988).
Additionally, to address the impact of reduced soil moisture and weed competition on corn photosynthetic response, corn biomass, and weed biomass, linear mixed effects models were constructed using the lmer function from the lme4 package in R (R Core Team 2021). Weed pressure, corn competition, and soil VWC were considered fixed effects, and replication and run were considered random effects. Differences in means were further investigated using Tukey’s HSD post hoc test in the emmeans package in R (R Core Team 2021).
Results and Discussion
Chenopodium album
Height
Corn competition, increasing weed pressure, and water stress did not decrease the days needed to reduce height by 10% or 20% (Table 2). Interestingly, with 6 weeds pot−1, without corn competition, under 50% VWC, C. album plants reached a 40% reduction in height 3 d faster than under 100% VWC (P = 0.07; Table 2). However, overall, holding C. album density constant, without corn competition, decreasing VWC did not decrease the days needed to reduce height by 40% (Table 2). Furthermore, holding C. album density constant, with corn competition, decreasing VWC did not affect the number of days needed to reduce C. album height by 40% (Table 2). Without corn competition, C. album plants grown under 50% VWC and 4 plants pot−1 reached a 40% reduction in height 4.2 d faster than C. album plants grown under 100% VWC and 6 plants pot−1 (P = 0.0003; Table 2). Furthermore, C. album plants grown under 9 plants pot−1, no corn competition, and 50% VWC reached a 40% reduction in height 2.9 d faster than C. album plants grown under the same density, but with the addition of corn competition and 100% VWC (P = 0.02; Table 2).
Table 2. Mean (SE) days required to reduce Chenopodium album height and leaf number by 10%, 20%, and 40% under two drought-tolerant corn densities, four weed pressures, and two soil volumetric water content (VWC) levels in a greenhouse study. a

a Percent reductions and probability values were calculated by ED and EDcomp functions, respectively, in the drc package in R (R Core Team 2021). Percent reduction data are days relative to the control of no corn competition, two weeds, and 100% VWC. Means within the same column followed by the same lowercase letter are not statistically different (P ≥ 0.1).
Overall, these results demonstrate that reducing the available water for C. album growth is a larger stress than increasing inter- or intraspecific competition under ample water conditions. To our knowledge, no study has been conducted to evaluate water stress and drought-tolerant crop competition on weed height. However, Maganti et al. (Reference Maganti, Weaver and Downs2005) reported that C. album height was reduced 29% to 55% with no crop competition under drought conditions. Additionally, Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2016) reported that decreasing VWC by 50% decreased common waterhemp (Amaranthus rudis Sauer) height by 10% compared with no water stress without crop competition.
Leaf Number
Corn competition, increasing weed pressure, and water stress did not decrease the days needed to reach a 10% reduction in leaf number (Table 2). Holding C. album density constant, with or without corn competition, reducing VWC by 50%, had no impact on the days needed to reduce leaf number by 20% (Table 2). However, without corn competition, C. album plants grown under 4 weeds pot−1 and 50% VWC reached a 20% reduction in leaf number 2.6 and 2.2 d faster than C. album grown under 6 or 9 weeds pot−1 and 100% VWC (P = 0.02 and 0.07; Table 2). Interestingly, C. album plants grown under 4 weeds pot−1, corn competition, and 50% VWC, reached a 20% reduction in total leaf production 2.9 d faster than C. album plants grown under 9 weeds pot−1, corn competition, and 100% VWC (P = 0.07; Table 2). Additionally, C. album plants grown under 4 plants pot−1, no corn competition, and 50% VWC reached a 20% reduction in height 2.9 d faster than C. album plants grown under the same density, but under corn competition and 100% VWC (P = 0.02; Table 2). Furthermore, C. album plants grown under 9 plants pot−1, no corn competition, and 50% VWC reached a 20% reduction in height 2.9 d faster than C. album plants grown under the same density, but under corn competition and 100% VWC (P = 0.09; Table 2).
Holding weed pressure constant, with no corn competition, and decreasing VWC by 50% did not affect the time needed to reach a 40% reduction in C. album leaf number (Table 2). However, C. album plants grown under 4 weeds pot−1, crop competition, and 50% VWC reached a 40% reduction in leaf number 4 d faster than C. album plants grown under the same crop and weed density, but under 100% VWC (P = 0.03; Table 2). Additionally, holding weed pressure constant at 9 weeds pot−1, increasing VWC by 50%, and adding corn competition increased the time required to reach a 40% reduction by 5 d (P = 0.02; Table 2). A similar trend occurred with 4 weeds pot−1, resulting in an increase in the time required to reach a 40% reduction in leaf number by 7.4 d (P = < 0.0001; Table 2). Finally, without crop competition, C. album plants grown under 4 weeds pot−1 and 50% VWC reached a 40% reduction in leaf number 4.4 and 5.8 d faster than C. album grown under 6 and 9 weeds pot−1 and 100% VWC (P = < 0.0001 and < 0.0001; Table 2).
Overall, these results demonstrate that introducing water stress decreases the rate of C. album leaf production. To our knowledge, no study has been conducted to evaluate water stress and drought-tolerant crop competition on C. album leaf production. Additionally, our results highlight that increasing weed or crop competition had little impact on overall leaf production compared with water stress. Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2016) reported that decreasing soil moisture by 50% decreased A. rudis leaf production by 30% compared with no water stress without crop competition.
Biomass
Reducing VWC by 50% did not reduce C. album biomass averaged across levels of corn and weed competition (P = 0.18). However, there was a significant two-way interaction between corn competition and weed pressure (P = 0.05). Without corn competition, increasing weed pressure from 2 to 4 weeds pot−1 did not reduce weed biomass (P = 0.98). However, without corn competition, increasing weed pressure from 2 weeds pot−1 to 6 and 9 weeds pot−1 decreased weed biomass by 44% and 56% (P = 0.0005 and <0.0001; Figure 1). Furthermore, without corn competition, increasing weed pressure from 4 weeds pot−1 to 6 and 9 weeds pot−1 decreased weed biomass by 38% and 51% (P = 0.01 and 0.0002; Figure 1). Increasing weed pressure from 6 to 9 weeds pot−1 did not negatively impact weed biomass (P = 0.95; Figure 1).
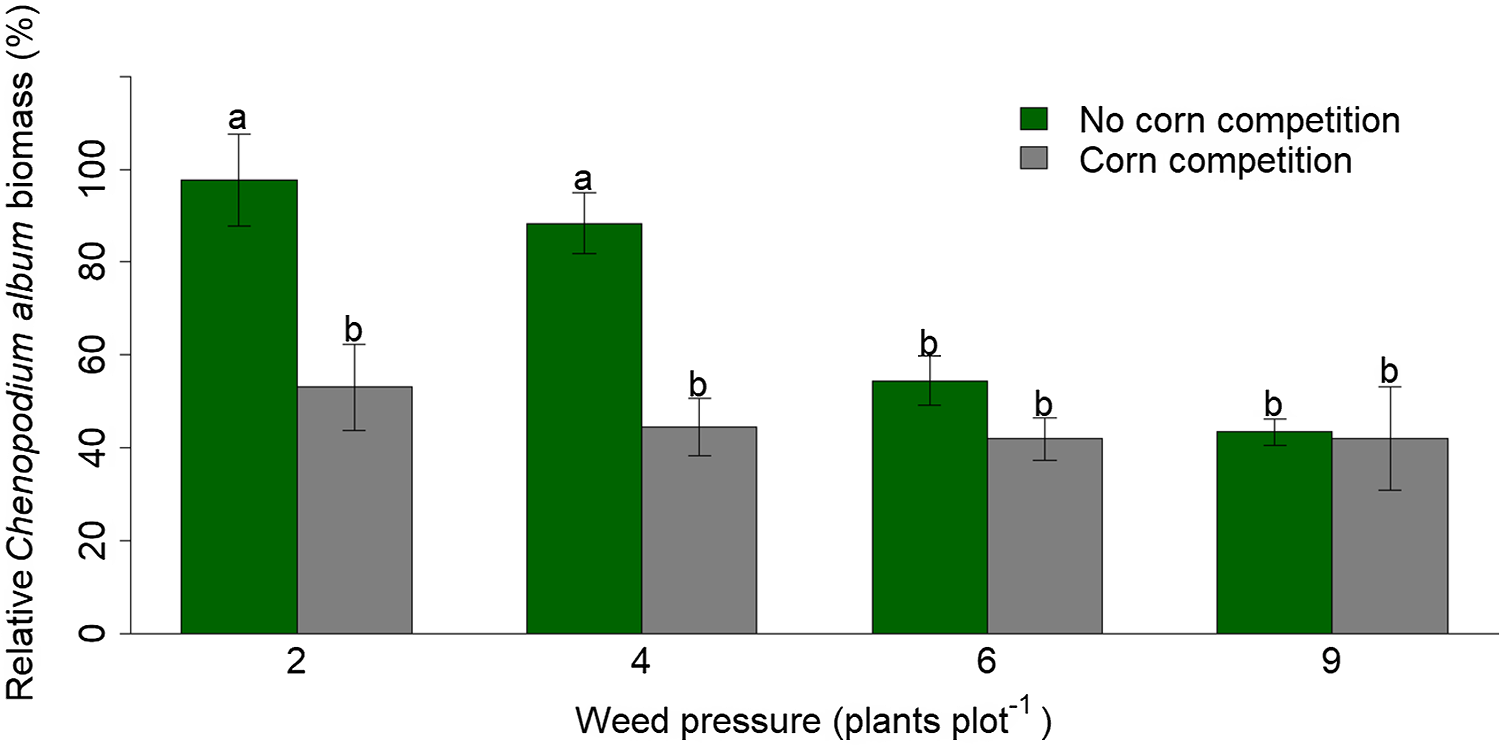
Figure 1. Mean (SE) relative Chenopodium album biomass impacted by drought-tolerant corn competition, four weed pressures, and averaged across two (50% and 100%) soil volumetric water contents (VWC) in a greenhouse study. Biomass reduction data are relative to the control of no corn competition, two weeds, and 100% VWC. Bars labeled by the same lowercase letter are not statistically different (P ≥ 0.05).
Under corn competition, increasing weed pressure did not reduce weed biomass (P ≥ 0.05; Figure 1). Additionally, C. album biomass was reduced by 46% and 50% when corn competition was introduced under 2 and 4 weeds pot−1, respectively (P = 0.0003 and 0.0004; Figure 1). However, introducing crop competition under 6 and 9 weeds pot−1 did not reduce C. album biomass (P = 0.90 and 1.00; Figure 1). Overall, these results demonstrate that increasing corn competition does not reduce weed biomass under high weed pressures but does decrease weed biomass under lower weed pressures. These findings support the results previously discussed regarding C. album height and leaf number. Similar results were reported by Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2016), in which A. rudis plant height, leaves per plant, and aboveground biomass decreased when VWC was reduced by 75%, without corn competition. However, Chahal et al. (Reference Chahal, Irmak, Jugulam and Jhala2018) reported that reduced soil moisture did not impact Palmer amaranth (Amaranthus palmeri S. Watson) leaf production but did reduce aboveground biomass by 35% compared with the control of no water stress.
Furthermore, we hypothesize that if C. album plants were allowed to grow until maturity, results similar to those of Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2016) would be observed. Therefore, future research should be conducted for a longer duration using larger pots to encompass C. album’s complete life cycle to confirm our study’s findings. Overall, it is evident that water stress plays a larger role in C. album physiological development (height and leaf production) than weed or crop competition but has little impact on biomass during the first 21 d of growth.
Corn
Height
Increasing weed pressure and reducing VWC by 50% did not decrease the number of days required to reduce corn height by 10% (Table 3). However, corn in competition with 2 (P = 0.007), 6 (P = 0.004), and 9 (P = 0.07) weeds pot−1 under 100% VWC reached a 20% reduction in height 2.1, 2.6, and 2.8 d faster than under 50% VWC, respectively (Table 3). Additionally, corn grown without weed competition under 100% VWC reached a 20% reduction in height 1.8, 2.0, and 3.7 d faster than corn grown under 50% VWC and 2 (P = 0.03), 4 (P = 0.05), or 6 (P = 0.004) weeds pot−1 (Table 3). Furthermore, corn in competition with 6 weeds pot−1 under 50% VWC reached a 20% reduction in height 2.6 and 2.9 d later than corn in competition with 4 (P = 0.03) and 9 (P = 0.009) weeds pot−1 under 100% VWC (Table 3).
Table 3. Mean (SE) days required to reduce drought-tolerant corn height and growth stage by 10%, 20%, and 40% under four weed pressures and two soil volumetric water content (VWC) levels in a greenhouse study. a

a Percent reductions and probability values were calculated by ED and EDcomp functions, respectively, in the drc package in R (R Core Team 2021). Percent reduction data are days relative to the control of one corn, no weed competition, and 100% VWC. Means within the same column followed by the same lowercase letter are not statistically different (P ≥ 0.1).
Holding weed competition constant at 0 (P = 0.68), 2 (P = 0.13), and 4 (P = 0.46) weeds pot−1 and decreasing VWC by 50% did not modify the number of days required to reach a 40% reduction in height (Table 3). However, holding weed competition constant at 6 (P = 0.03) and 9 (P = 0.07) weeds pot−1 and decreasing VWC by 50% increased the number of days required to reach a 40% reduction in height by 17.2 and 21 d, respectively (Table 3). Additionally, decreasing VWC by 50% and increasing weed pressure from 2 to 6 weeds pot−1 (P = 0.01) and 4 to 9 weeds pots−1 (P = 0.02) increased the number of days required to reach a 40% reduction in height by 18 and 26 d, respectively (Table 3).
Drought impacts on corn growth are well documented (Cakir Reference Cakir2004; Ge et al. Reference Ge, Sui, Bai, Tong and Sun2012; Licht and Archontoulis Reference Licht and Archontoulis2017). From our results, drought-tolerant corn reaches 20% and 40% reduction in height faster under 100% than 50% VWC, which suggests that under 50% VWC, drought-tolerant corn buffers weed competition better than under 100% VWC. To our knowledge, no study has been conducted to evaluate water stress and weed competition on drought-tolerant corn height. However, Licht and Archontoulis (Reference Licht and Archontoulis2017) reported that water stress during the vegetative growth stages reduces stem and leaf cell expansion, resulting in shorter plants. Additionally, Cakir (Reference Cakir2004) reported that water stress during the early vegetative growth stages of corn decreases corn height. Furthermore, research by Ge et al. (Reference Ge, Sui, Bai, Tong and Sun2012) reported that in the field among three increasing levels of water-stress treatments (33%, 55%, and 75% VWC), differences in plant height were not detected at 21 d after planting. In contrast, our results demonstrate that corn height is reduced within 2 wk of water-stress initiation (Table 3).
Growth Stage
Increasing weed pressure and water stress did not impact the number of days needed to reduce corn growth stage by 10%, 20%, or 40% (Table 3). Additionally, averaged across weed pressures and VWCs, 10%, 20%, and 40% reductions in corn growth stage occurred at approximately 2, 3, and 6 d after water-stress initiation (Table 3). Our results are supported by Hatfield and Prueger (Reference Hatfield and Prueger2015), who reported that increasing the temperature by 4 C during corn pollination had no impact on leaf collar development among three water treatments (50%, 100%, and 125% of the normal precipitation patterns). Although Hatfield and Prueger (Reference Hatfield and Prueger2015) reported that corn leaf collars were not impacted, grain fill was reduced, which can reduce final crop yield.
Biomass
Averaged across weed pressures, corn biomass was reduced by 22% when grown under 50% VWC compared with 100% VWC (P = 0.0003; Figure 2). Additionally, averaged across VWC values, there was no difference in corn biomass when grown with 2, 4, 6, and 9 weeds pot−1 (Figure 2). However, averaged across VWC values, increasing weed competition from 0 to 2 (P = 0.04), 4 (P = < 0.0001), 6 (P = 0.0002), or 9 (P = 0.0002) weeds pot−1 reduced biomass by 22%, 38%, 35%, and 36% (Figure 2).

Figure 2. Mean (SE) relative drought-tolerant corn biomass impacted by four weed pressures and two soil volumetric water content (VWC) levels in a greenhouse study. Bars labeled by the same capital letter are not statistically different for the main effect of VWC (P ≥ 0.05). Bars labeled by the same lowercase letter are not statistically different for the main effect of weed pressure (P ≥ 0.05). Biomass reduction data are relative to the control of 1 corn plant pot−1, no weed competition, and 100% VWC.
Overall, these results indicate that reduced soil moisture and high weed densities decrease drought-tolerant corn biomass. However, the magnitude of drought-tolerant corn biomass reduction is stronger under drought stress than increasing levels of weed competition when water is not limiting. Ge et al. (Reference Ge, Sui, Bai, Tong and Sun2012) reported corn biomass was reduced by 68% when grown under water stress (55% reduction in soil moisture). The reduction we observed in this study was not as severe; potential reasons include the length of the study (21 vs. 70 d) and field versus greenhouse conditions. Specifically, our study evaluated impacts of drought and weed competition on vegetative growth, which had not been previously evaluated; however it is well known that drought during the reproduction growth stage of silking can cause yield decreases of 55% (Claassen and Shaw Reference Claassen and Shaw1970). Additionally, crop–weed interactions will vary depending on other altered climatic conditions not evaluated in this study, including temperature and soil type (Singh et al. Reference Singh, Singh and Singh2011). Photosynthetic pathways also play a role in weed competitiveness under water stress. For example, C4 species such as kochia [Bassia scoparia (L.) A. J. Scott] and Russian thistle (Salsola tragus L.) have been found to be highly competitive under reduced soil moistures (Wiese and Vandiver Reference Wiese and Vandiver1970). In contrast, C. album used in this study is a C3 broadleaf summer annual weed common to cropping systems in the Great Lakes Region (Korres et al. Reference Korres, Norsworthy, Tehranchian, Gitsopoulos, Loka, Oosterhuis, Gealy, Moss, Burgos and Miller2016).
Photosynthesis
Neither increasing weed pressure (P = 0.58) and water stress (P = 0.38) nor their interaction (P = 0.68) negatively impacted Phi2 levels at 14 d after treatment (Table 4). However, there was a significant main effect of weed pressure (P = 0.03) and VWC (P = 0.05) on Phi2 levels at 21 d after treatment. Furthermore, at 21 d, decreasing VWC by 50% reduced corn Phi2 levels by 6.6% compared with 100% VWC averaged across weed densities (P = 0.05; Table 4). Additionally, at 21 d, increasing weed pressure from 0 weeds pot−1 to 4 (P = 0.02) or 6 (P = 0.05) weeds pot−1 increased Phi2 levels by 16% and 15%, respectively, averaged across VWC values (Table 4). Phi2, or quantum yield of photosystem II photochemistry, is the percentage of incoming light that goes into photosystem II, which is a measure of photosynthetic efficiency (Kramer et al. Reference Kramer, Johnson, Kiirats and Edwards2004). Under water-limiting conditions, results from this study demonstrate that drought-tolerant corn photosynthetic efficiency is reduced. Previous research has also concluded that drought stress significantly reduced Phi2 levels in cowpea [Vigna unguiculata (L.) Walp.] (Mwale et al. Reference Mwale, Ochwo-Ssemakula, Sadik, Achola, Okul, Gibson, Edema, Singini and Rubaihayo2017). However, increasing weed competition increased the percentage of light entering photosystem II, therefore suggesting that drought-tolerant corn hybrids may tolerate early-season weed competition by increasing the amount of energy entering photosystem II.
Table 4. Mean (SE) drought-tolerant corn photosynthetic response to four weed pressures and two soil volumetric water content (VWC) levels at 14 and 21 d after water-stress initiation in a greenhouse study. a

a Means within the same column followed by the same capital letter are not significantly different (P ≥ 0.1) for the main effect of VWC. Means followed by the same lowercase letter are not significantly different (P ≥ 0.1) for the main effect of weed pressure.
b Phi2, quantum yield of photosystem II; PhiNPQ, quantum yield of non-photochemical quenching; PhiNO, quantum yield of other unregulated losses.
Neither increasing weed pressure (P = 0.69) and water stress (P = 0.26) nor their interaction (P = 0.71) negatively impacted PhiNPQ levels at 14 d after treatment (Table 4). However, there was a significant main effect of weed pressure (P = 0.05) and VWC (P = 0.02) on PhiNPQ levels at 21 d after treatment. Furthermore, at 21 d, decreasing VWC by 50% increased corn PhiNPQ by 20% compared with 100% VWC averaged across weed densities (P = 0.02; Table 4). However, at 21 d, increasing weed competition from 0 weeds pot−1 to 4 (P = 0.05) or 6 (P = 0.09) weeds pot−1 decreased PhiNPQ levels by 28% and 25%, respectively, averaged across VWC values (Table 4). PhiNPQ, or quantum yield of non-photochemical energy, measures energy loss via downregulation of photochemistry (Kramer et al. Reference Kramer, Johnson, Kiirats and Edwards2004). These results are in accordance with those for Phi2, in which drought reduced photosystem II efficiency, thus leading to an increase in energy loss. Furthermore, the reduction in energy loss under increasing weed density is supported by the increase in photosystem II efficiency discussed earlier.
Neither increasing weed pressure (P = 0.99) and reducing VWC by 50% (P = 0.21) nor their interaction (P = 0.59) negatively affected PhiNO levels at 14 d after treatment (Table 4). However, at 21 d after treatment, the main effect of VWC was significant (P = 0.008), but the main effect of weed pressure (P = 0.30) or the interaction of VWC and weed pressure (P = 0.47) were not significant. Specifically, reducing VWC by 50% (P = 0.008) decreased PhiNO levels by 9%, averaged across weed densities (Table 4). PhiNO is the quantum yield of other unregulated processes (Kramer et al. Reference Kramer, Johnson, Kiirats and Edwards2004). Unlike Phi2 and PhiNPQ, increasing weed competition had no impact on PhiNO levels at 21 d after treatment.
Overall, the PhotosynQ instrument allows for the evaluation of sensitive indicators of various photosynthetic parameters and the ability to view the onset of photoinhibition and photodamage that may be caused by plant stress (Baker and Rosenqvist Reference Baker and Rosenqvist2004). To our knowledge, no study has been conducted to evaluate photosynthetic parameters on drought-stressed drought-tolerant corn hybrids and increasing weed competition using the PhotosynQ instrument. However, previous research has been conducted on the photosynthetic response of drought-stressed drought-tolerant corn hybrids using a LI-COR meter. In a greenhouse study conducted by Wijewardana et al. (Reference Wijewardana, Henry and Reddy2017), photosynthetic rates measured five times during the study declined in drought-tolerant corn hybrids as soil moisture levels decreased. However, our results demonstrate that for the first 14 d of growth, reduced soil moisture and weed competition do not modify corn photosynthesis; but after 21 d, reduced soil moisture decreased Phi2 and PhiNO levels, while increasing PhiNPQ. In contrast, increasing weed pressures increased Phi2 while decreasing PhiNPQ and had no impact on PhiNO levels. Future research should evaluate whether the modification of photosynthetic efficiency in vegetative drought-tolerant corn by drought and weed competition reported here, would also occur at later growth stages to fully understand how reduced soil moisture and weed competition impacts drought-tolerant corn photosynthesis.
Overall, nearly all crop production is impacted by precipitation patterns. Future climate scenarios for the Great Lakes Region predict more precipitation falling in heavy rainfall events, leaving more days during the growing season that have little or no precipitation, polarizing the wet and dry periods. Given these projections, drought events may become more common. Results from this study highlight that soil moisture stress plays a larger role in reducing C. album height and leaf number than drought-tolerant corn competition. However, drought-tolerant corn responds differently to reduced soil moisture than C. album. Interestingly drought-tolerant corn in competition with high weed densities reached a 40% reduction in height faster when under 100% rather than 50% VWC. This finding can be interpreted in one of two ways: (1) C. album is only competitive when soil moisture is abundant; or (2) drought-tolerant corn is more competitive than C. album under reduced soil moisture and thus able to buffer the additional stress from weed competition. Biomass results may help elucidate these results. Specifically, drought-tolerant corn competition had no impact on C. album biomass at high weed densities but did decrease C. album biomass at low weed densities. We attribute this difference to the fact that there is already so much stress occurring at the higher weed densities that the additional stress of corn competition has little additional negative impact on biomass. Interestingly, reduced soil moisture decreased drought-tolerant corn Phi2 levels, but increased PhiNPQ levels, thus leading to net energy loss. However, due to this study only being conducted for 21 d, future research should be conducted until natural plant senescence to fully understand how reduced soil moisture and weed competition impact growth and photosynthetic parameters of a drought-tolerant corn hybrid. However, early-season weed competition has been documented to have dramatic effects on crop physiology and biochemical response; this research adds to that literature (McKenzie-Gopsill et al. Reference McKenzie-Gopsill, Lee, Lukens and Swanton2016, Reference McKenzie-Gopsill, Amirsadeghi, Earl, Jones, Lukens, Lee and Swanton2019). Additionally, future research should include multiple levels of water stress outside those measured in this study and should also evaluate different durations of water stress.
This study was the first step in identifying how climate will influence weed biology and drought-tolerant corn growth and competitive capacity. Although genetic improvements to corn have been made, resulting in corn with increased levels of drought tolerance, it is evident weed competition under reduced soil moisture conditions will detrimentally impact yield. Ultimately, taking proactive integrated weed management steps by using the corn–weed competition principles investigated in this study will allow field crop growers across the Great Lakes Region to integrate weed biology and crop physiology in the development of integrated economically viable weed management programs under future climate stress.
Acknowledgments
This project was supported by Project GREEEN through Michigan State University. No conflicts of interest have been declared. A special thank you to the Burns Weed Science Lab for assisting in greenhouse watering, data collection, and harvest.











