CVD, a leading cause of the global burden of death and disease in adults(Reference Roth, Johnson and Abajobir1,Reference Mendis, Armstrong and Bettcher2) , is attributed to a number of predisposing factors including low physical activity and an unhealthy dietary pattern(Reference Siri-Tarino and Krauss3,Reference Vos, Kaar and Welsh4) . Recent research suggests that a major contributor of unhealthy dietary patterns is a high intake of ultra-processed foods (UPF)(Reference Monteiro, Cannon and Lawrence5). Moreover, it has been reported that risk factors driving CVD, such as high serum lipid concentrations, have their origins even in early childhood(Reference Juhola, Magnussen and Viikari6,Reference Steinberger, Daniels and Hagberg7) . High serum lipid profiles are directly affected by a poor diet, despite the fact that diet is one of the most important modifiable risk factors in the prevention of chronic non-communicable diseases(8–Reference de Oliveira Otto, Afshin and Micha10). However, an area that has received limited attention relative to UPF intake and lipid profiles in childhood is the focus of this paper.
The global consumption of UPF, characterised as ready-to-eat and energy-dense manufactured foods, has increased dramatically(Reference Monteiro, Moubarac and Levy11–Reference Monteiro, Levy and Claro14). Sales of UPF in low- and middle-income countries are rising at a disproportionate rate compared with high-income countries(Reference Vandevijvere, Jaacks and Monteiro15). For example, Euromonitor reported a sales growth in UPF by 30 % in Brazil from 2000 to 2013, while in the same period, sales dropped in the USA and Canada (–9 and –7·3 %, respectively)(16). Several studies have shown that high contribution of UPF in dietary patterns is associated with diets of lower nutritional quality(Reference Louzada, Ricardo and Steele17–Reference Martínez Steele, Popkin and Swinburn19) assessed by NOVA, a classification of foods based on their degree and purpose of industrial food processing. These findings are especially important given that dietary patterns in this childhood often track into adulthood(Reference Ventura and Worobey20).
Several studies have been conducted to quantify the burden of disease attributable to specific UPF consumption. In adults, consumption of UPF has been associated with an increased risk of overweight and obesity(Reference Mendonça, Pimenta and Gea21–Reference Juul, Martinez-Steele and Parekh23), and related conditions, such as hypertension(Reference Mendonça, Lopes and Pimenta24), CVD(Reference Srour, Fezeu and Kesse-Guyot25), as well as higher risk of overall cancer(Reference Fiolet, Srour and Sellem26). However, few studies focus on the association between consumption of UPF and risk of diseases in childhood(Reference Rauber, Campagnolo and Hoffman27–Reference Machado Azeredo, Cortese and Costa29). It is critical to better understand the possible effects of these changing patterns, in particular how they may influence metabolic risk factors for non-communicable diseases in the paediatric population. Therefore, the objective of our longitudinal study was to assess trends of UPF consumption and determine the association between UPF and lipid profile in a population of children in southern Brazil.
Methods
Study design
This longitudinal study used data from children at 3 and 6 years of age who participated in a randomised intervention study (NCT00635453) of breast-feeding and dietary practices(Reference Vitolo, Louzada and Rauber30). The intervention trial was conducted in health centres that provide primary care services predominantly to low-income families in Porto Alegre, Brazil, and the outcomes were assessed for mothers and children who received treatment at these centres. Briefly, health workers (physicians, nurses and administrative staff) of all intervention health centres participated in a training based on the ‘Ten Steps for Healthy Feeding for Brazilian Children from Birth to Two Years of Age’ guideline and strategies to provide suggestions how best to incorporate recommendations into the consultations. From April to December 2008, all pregnant women in the last trimester at the participating health centres were invited to sign up for outcome tracking by interviewers who were blinded to the allocation status of the participants. All births occurred between May 2008 and February 2009.
The sample size initially chosen for the trial was based on the goal of detecting a difference in the prevalence of exclusive breast-feeding at 4 months(Reference Vitolo, Bortolini and Feldens31). A power of 90 %, α of 0·5, design effect of 1·5 and a loss prediction of 20 % were used to calculate the sample size, resulting in the inclusion of 360 mother–baby pairs in the intervention group and another 355 in the control group. Of 715 pregnant women who registered initially, 635 of their children were enrolled at the study at 6 months of age. A total of 476 and 387 children at 3 and 6 years of age, respectively, underwent assessment in the follow-up study. As the present study had a different aim, we proceeded to estimate if the available sample at age 6 years was sufficient to investigate the association between UPF consumption and outcomes. Assuming a correlation coefficient in the order of 0·3 and a design effect of 2, a total sample size of 226 children was required (with 90 % power and α = 5 %). This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the Universidade Federal de Ciências da Saúde de Porto Alegre. Informed consent was obtained from mothers on behalf of their children at each stage of data collection.
Data collection
Mothers were interviewed at home visits by trained interviewers when their children were at 6 months, 3 years and 6 years of age. Home visits for data collection by the interviewers were verified through telephone callbacks to a 5 % random sample of interviewed mothers. Socio-economic and family characteristics were obtained at recruitment. Birth weight and length, and sex were collected from the children’s health records. Pre-pregnancy weight was self-reported, and mothers’ height was measured during home visits when the children were 6 months of age and pre-pregnancy BMI was calculated. Anthropometric data of all children were obtained at 3 and 6 years using a digital scale to the nearest 0·1 kg and a stadiometer to the nearest 0·1 cm. BMI for age z-score was calculated based on the WHO standards(32), and obesity was defined as BMI z-score >2 sd for all ages.
Dietary assessment
Two multiple-pass 24-h dietary recalls were collected for each child at ages 3 and 6 years during the home visits on two non-consecutive days that were chosen randomly within 2 weeks to 1 month(33). For children at age 3 years, recalls were provided by mothers or other caregivers; at age 6 years, children reported all the foods and beverages consumed the day before the interview, with help from their parents. For children who spent time with a caregiver other than the parent (e.g. during school hours), we contacted the caregiver to record all items the children consumed during the previous day. When it was not possible to obtain all available information, the participants were contacted on a different day to administer that 24-h recalls to provide accurate data for all children. Details about food types, amounts and preparation methods were recorded. Common household measures (e.g. teaspoons, tablespoons, cups and serving sizes) were used to help mothers report the amounts of food given to their children and to standardise portion sizes. Interviews were conducted by dietitians/nutritionists and undergraduate students in nutritional sciences trained and supported in the 24-h recall method with standardised procedures, including practice interviews prior to the start of the study. The research supervisor reviewed all the dietary recalls. Dietary energy intake was estimated using the Dietwin® software programme (version 2008 professional Dietwin®), and the Brazilian Food Composition Table (TACO, 2006) was preferentially used as a reference, followed by the United States Department of Agriculture chemical composition tables (Agricultural Research Service, 1998). For commercial products, we manually added all nutritional composition provided by the manufacturer to the programme.
Ultra-processed food consumption
UPF was assessed using the NOVA classification system(Reference Monteiro, Cannon and Levy34,Reference Monteiro, Cannon and Moubarac35) , a four-group food classification based on the extent and purpose of food processing, including unprocessed and minimally processed foods, processed culinary ingredients, processed foods and UPF. This study focused on the NOVA group of UPF. Briefly, UPF are formulations of ingredients, most of exclusive industrial use, that result from a series of industrial processes and typically including little or no fresh food. UPF are ready to eat, drink or heat (e.g. soft drinks, sweet or savoury packaged snacks, breakfast cereal, candies, chocolate, ‘instant’ soups and noodles, processed meats, pre-prepared frozen dishes; and many other products). All food and drink items assessed in the dietary surveys were categorised as ultra-processed or not based on the food classification previously reported. This categorisation was performed by a team of dietitians trained, supervised by researchers. Home-made recipes were identified and decomposed using standardised recipes, and the classification was applied to their ingredients. For a small number of specific food items such as pizza, there was insufficient information for classification purposes. In those cases, we used a conservative approach, such that the lower level of processing was chosen. UPF subcategories analysed in the present study included (1) savoury and biscuits (crackers, chips, salty snacks, cookies), (2) soft drinks (soda, sweetened juice), (3) sweets (candies, chocolate and ice cream), (4) powdered chocolate, (5) sugary milk beverages, (6) processed meats, (7) breads, (8) baby cereal, (9) margarine, mayonnaise and dressing and (10) ready-made soup/noodle (instant noodle and dehydrated soup).
Lipid profile
Venous blood samples were collected at 6 years of age to assess serum lipid profile, and analyses were performed at the laboratory of the Universidade Federal de Ciências da Saúde de Porto Alegre. Total cholesterol (TC), HDL-cholesterol and TAG levels were measured using standard enzymatic methods with an automatic analyzer (BS-120, Mindray). LDL-cholesterol was calculated according to Friedewald’s formula(Reference Friedewald, Levy and Fredrickson36). Cut-off values for abnormal lipid concentrations were defined according to the American College of Cardiology/American Heart Association (TC ≥ 4·40 mmol/l, LDL ≥ 2·85 mmol/l, HDL < 1·17 mmol/l and TG ≥ 0·85 mmol/l)(Reference Grundy, Stone and Bailey37).
Data analysis
The usual dietary energy intake of UPF was estimated by the Multiple Source Method (https://msm.dife.de)(Reference Harttig, Haubrock and Knüppel38) that calculates dietary intake for individuals and then constructs the population distribution based on these data(Reference Laureano, Torman and Crispim39,Reference Haubrock, Nöthlings and Volatier40) . All participants were considered consumers of total energy intake and for UPF groups; a probability value of 0·5 (50 %) was used to assign the status of habitual consumer. Contribution of each subgroup of UPF to the total energy intake was calculated as a percentage of total energy.
Differences between intervention and control groups with respect to UPF consumption were analysed, and no differences at 3 (P = 0·697) and 6 (P = 0·606) years of age were observed. Continuous variables were expressed as mean and standard deviation (normally distributed data) or median and interquartile range (non-normally distributed data) and percentage frequency. Analyses of trends in UPF consumption from age 3 to 6 years were conducted using Wilcoxon signed pair test, and the percentage change across study period was evaluated.
Crude and multivariable linear regression models were used to determine the relationship between consumption of UPF at age 3 years and lipid profiles at age 6 years. All models were adjusted for sex, group status in early phase (intervention or control), birth weight, pre-pregnancy BMI and family income (model 1), in addition to BMI z-score at baseline (3 years) (model 2) and for total energy intake and percentage of fat intake at age 3 years (model 3). The lowest tertile was used as the reference group, and differences between strata of UPF consumption are presented as standardised regression coefficients with 95 % CI. We also estimated the change in lipid levels for a 10 % increase in UPF consumption (continuous variable). Tests of linear trend were performed in all models by tertiles of UPF consumption as a continuous variable. All statistical analyses were conducted using SPSS version 21.0 (IBM Statistics Inc.), and statistical significance was set at P < 0·05.
Results
Of the 633 originally infants at 6 months of age included in the study, 476 and 387 children underwent assessment at the mean age of 3 and 6 years, respectively. The main reasons for loss were refusal to participate in the study and change of address (Fig. 1). No differences were found for sex, birth weight, maternal age at child’s birth and family income between the children who were lost to follow-up and those who remained at 6 years of age (P > 0·05; data not shown). The final analysis included 308 children with both complete dietary at age 3 years and blood data at age 6 years.

Fig. 1. Flow diagram of the study.
Characteristics of the study participants are presented in Table 1. Briefly, 18·9 % of the mothers were under age 20 years at the child’s birth and 28·9 % had 8 years of schooling or less. Family income was low for most families – 62·5 % had a monthly income less than three times the national minimum wage (approximately $804·00/month). More than half of the children were male (52·0 %), and the obesity prevalence was 18 % at age 3 years and 18·4 % at age 6 years. Descriptive information of the lipid profile is shown in Table 2. Among children at age 6 years, prevalence of abnormal concentrations of TC, TAG, LDL-cholesterol and HDL-cholesterol was 39·9, 36·0, 14·0, and 8·8 %, respectively.
Table 1. Characteristics of children and their families (n 308)* (Numbers and percentages; mean values and standard deviations)

* Values may not equal to total number of subjects in each group because of missing data.
Table 2. Prevalence of alterations in lipid profile at age 6 years (n 308) (Numbers and percentages; mean values and standard deviations)
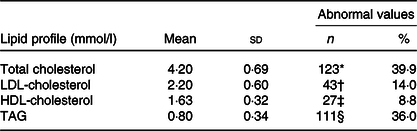
* ≥4·40 mmol/l.
† ≥2·85 mmol/l.
‡ <1·17 mmol/l.
§ ≥0·85 mmol/l.
Consumption of UPF represented a median of 43·4 and 47·7 % of the total energy intake at 3 and 6 years of age, respectively (Table 3). Within food groups, the significant energy contributors were savoury and biscuits, soft drinks and sweets in both age groups. Taken together, these UPF provided 25·8 and 28·4 % of the total energy intake at age 3 and 6 years, respectively. The overall dietary contribution of UPF increased by 10 % (P < 0·001) during a mean follow-up period of 3 years. The largest relative increase among UPF groups occurred for margarine, mayonnaise and dressing (1200 %; P < 0·001). There were notable changes in processed meat (58·3 %; P < 0·001) and bread (19·3 %; P < 0·001) consumption during childhood. Decreasing intake was seen for sugary milk beverages (41·0 %; P < 0·001) and powdered chocolate (27·5 % P < 0·001) over the study period.
Table 3. Trends in the consumption of ultra-processed foods (UPF) (% of total energy) at ages 3 and 6 years old (Median values and interquartile ranges (IQR))
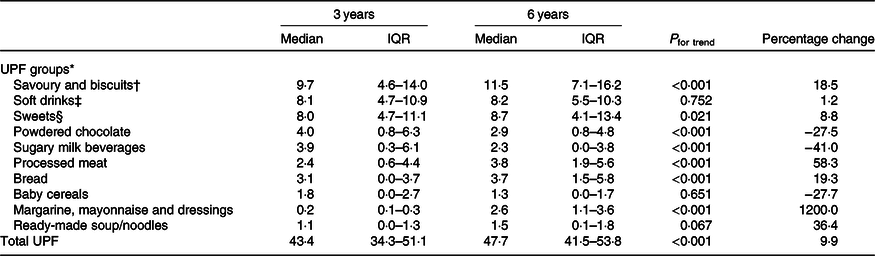
* Expressed as percentage of total energy intake.
† Crackers, chips, salty snacks, cookies.
‡ Soda, sweetened juice.
§ Candy, chocolate and ice cream.
Longitudinal associations between UPF intake and lipid profile are shown in Table 4. There was a positive association between UPF consumption at age 3 years and total serum cholesterol at age 6 years. In the fully adjusted model, higher UPF intake was associated with increased levels of total serum cholesterol later at age 6 years (tertile 3 v. tertile 1; β 0·22 mmol/l; 95 % CI 0·04, 0·39). Similarly, a 10 % increase in the consumption of UPF was associated with a 0·07 mmol/l (95 % CI 0·00, 0·14) increase in TC. Children in the highest tertile of UPF consumption had a mean TAG value of 0·11 mmol/l higher (95 % CI 0·01, 0·20) than those in the lowest tertile consumption in the fully adjusted model. Statistically significant associations were observed for an absolute increment of 10 in the percentage of UPF and TAG (β 0·04 mmol/l, 95 % CI 0·01, 0·07).
Table 4. Linear regression analyses of the association of ultra-processed food (UPF) consumption at 3 years old with lipid profile at 6 years old (β Values and 95 % confidence intervals)
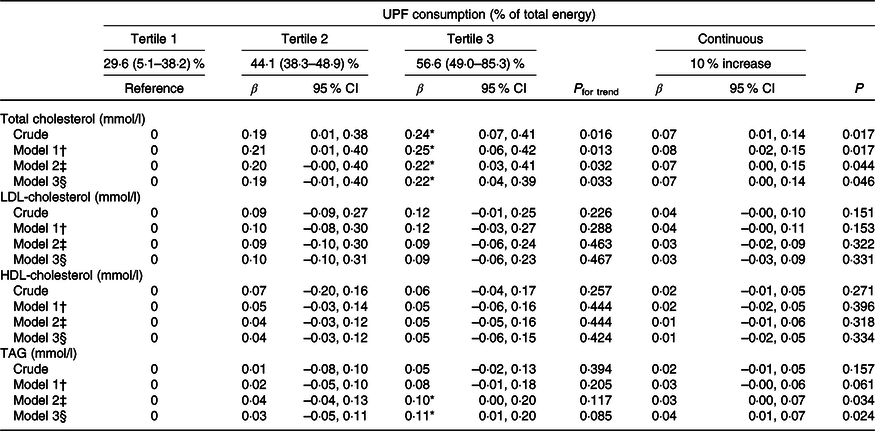
* Difference between the first and third tertile (P < 0·05).
† Model 1 = adjusted for sex, group status in the early phase (intervention and control), family income, pre-pregnancy BMI and child birth weight.
‡ Model 2 = model 1 + BMI z-scores at 3 years.
§ Model 3 = model 2 + intakes of total energy and total fat (%) at 3 years.
Discussion
While the global consumption of UPF has increased greatly, only a few studies have reported an association between UPF intake and health outcomes in children(Reference Rauber, Campagnolo and Hoffman27–Reference Machado Azeredo, Cortese and Costa29). Results from our longitudinal study suggest that higher UPF consumption during childhood was associated with higher levels of TC and TAG. In addition, we found that the consumption of UPF increased greatly during this period of early childhood. Overall, our results support the recent report of the FAO of the United Nations(Reference Monteiro, Cannon and Lawrence5) that highlights the need for studies analysing the impact of UPF as a risk factor for chronic non-communicable diseases in childhood.
Our data provide a comprehensive view of changes in UPF consumption from early to middle childhood. Briefly, UPF accounted for approximately 50 % of total energy consumed by the children, even for those in the lowest tertile of UPF intake, the consumption of these products was particularly high in this population compared with the general Brazilian population(Reference Louzada, Martins and Canella41). We also found a 10 % increase in total UPF consumption during the study period, suggesting a decline in diet quality the children studied. The increase in energy provided by the UPF is accompanied by a change in types of these products consumed, with the replacement of UPF targeting toddlers, such as ‘sugary milk beverages’ and ‘baby cereals’, by a dietary pattern based on ready-to-eat meals, such as sandwiches made with processed meat, bread and margarine, mayonnaise or dressing. Moreover, this shift in dietary patterns may be a result of complex social and environmental factors. For example, families in low-income urban neighbourhoods have limited availability of healthful food and a greater availability of UPF(Reference Pitt, Gallegos and Comans42–Reference Leite, de Carvalho Cremm and de Abreu44). Likewise, low-income groups, such as families enrolled in this study, are more likely to choose low-cost foods when available(Reference Darmon and Drewnowski45). Thus, providing detailed information about food consumption during childhood is necessary to develop effective strategies to prevent the chronic diseases later in life.
In regard to the development of chronic diseases, we found that higher consumption of UPF at age 3 years was significantly associated with higher blood lipid concentrations at age 6 years. There are a number of explanations for the results provided. For example, a high consumption of UPF is associated with unhealthful dietary patterns, characterised by an excess intake of energy, fat and added sugar(Reference Rauber, da Costa Louzada and Steele18,Reference Louzada, Martins and Canella41,Reference Neri, Martinez-Steele and Monteiro46) , especially among children and adolescents(Reference Rauber, Louzada and Martinez Steele47,Reference Cediel, Reyes and da Costa Louzada48) . Indeed, increased total energy intake, which is mainly driven by overconsumption of high-fat and high-sugar foods, is associated with increased lipogenesis and increased concentrations of circulating TAG and cholesterol(Reference Siri-Tarino and Krauss3). Moreover, it has been reported that an excess intake of dietary sugar may be a central mediator for de novo lipogenesis, stimulating overproduction of hepatic TAG, resulting in hypertriacylglycerolaemia(Reference Jacome-Sosa and Parks49,Reference Softic, Cohen and Kahn50) . In addition to nutrient level mechanisms, an excess intake of UPF is inversely associated with a lower intake of fruits and vegetables, foods that are known to prevent CVD(Reference Aune, Giovannucci and Boffetta51). Furthermore, given that obesity is a significant predictor of poor cardiometabolic health(Reference Freedman, Dietz and Srinivasan52,Reference Umer, Kelley and Cottrell53) , a high intake of UPF, which has occurred following aggressive advertising and marketing of UPF(Reference Fardet54,Reference Filgueiras, Pires de Almeida and Koch Nogueira55) , may promote obesity through a disruption of hunger and satiety(Reference Mallarino, Gómez and González-Zapata56,Reference Pulker, Scott and Pollard57) . Thus, these mechanisms support the association between UPF consumption and an unhealthy lipid profile, potentially increasing the risk of CVD.
Given the increased prevalence of CVD throughout the world(Reference Roth, Johnson and Abajobir1), our findings may be used to better elucidate the impact of UPF consumption on risk factors for CVD. For example, elevated TC and TAG are of critical significance since these results suggest an early vascular inflammatory response(Reference Steinberger, Daniels and Hagberg7,Reference Huet, Roubille and Roubille58) . As the intake of UPF can continue increasing in lifelong, there is a real potential that these products may later affect the metabolism of LDL- and HDL-cholesterol. It is worth noting that significant changes generally occur in lipoprotein metabolism following puberty(Reference Berenson, Srinivasan and Cresanta59), which could explain the absence of association between UPF consumption and LDL- and HDL-cholesterol in our study. A cohort study in France reported that an increment of 10 in the percentage of UPF consumption was associated with a 12 % increase in the rates of CVD in adults(Reference Srour, Fezeu and Kesse-Guyot25). A longitudinal study of 8-year-old Brazilian children found an association between consumption of UPF and higher levels of total and LDL-cholesterol(Reference Rauber, Campagnolo and Hoffman27), but not of higher TAG. Nonetheless, our results extend this work by studying younger children who lived in a major urban centre with greater access to UPF. The negative health risks of UPF consumption in the paediatric population are of particular concern as blood lipid profiles may worsen later in life as undesirable changes in eating behaviours are common during the transition from childhood to adolescence(Reference Birch, Savage and Ventura60). From a public health perspective, the robust evidence demonstrating the impact of UPF consumption on child health, investing in diet quality is one of the main priorities to promote cardiovascular health(Reference Fernandez-Jimenez, Al-Kazaz and Jaslow61).
There are potential limitations of our study that should be discussed to fully appreciate our results. First, a number of participants were lost during follow-up, but there were no significant differences between the baseline characteristics of children who remained in the study and those lost to follow-up. Second, cautious generalisation is required since the majority of our sample had low family income and may limit the applicability of our findings for more privileged populations. Third, the average of the two dietary recalls may not represent the entire distribution of usual intake due to the intra-individual variance component. Despite these limitations, our study has a number of strengths that merit attention. First, the prospective study design allowed us to assess the longitudinal association between UPF intake and lipid profile. Second, we collected detailed dietary data, including food preparation methods, ingredients used in dishes and the brand of commercial products, which allowed us to classify foods according to the NOVA system, a valid tool for public health and nutrition research and policy(16,62,63) .
In conclusion, we found that there has been a significant increase in the percentage of energy intake from UPF during childhood for children from a low-income community in Brazil. More important, we determined that a higher consumption of UPF was associated with increased blood lipids for the children in this study. The results of our study improve the understanding of how UPF intake may contribute to poor diet quality in this critical period of life. Thus, there is an urgent need for double- and triple-duty actions focused on minimising the consumption of UPF in early life to reduce the risk of CVD later in life.
Acknowledgements
The authors would like to thank the healthcare workers and families who participated in the study.
This work was supported by Brazilian Ministry of Health (no. 577/200), Research Support Foundation of the State of Rio Grande do Sul (PPSUS/2006/1537-7) and Brazilian National Council for Scientific and Technological Development (no 14/2013-47731/2013-8). Also, this study was supported by Coordination for the Improvement of Higher Education Personnel (PSL doctoral fellowship, no. 88881.362086/2019-01).
P. S. L. formulated the research question, analysed and interpreted the data, performed statistical analysis and wrote the manuscript. D. J. H. interpreted the data and statistical analysis and contributed to the drafting and critical revision of the manuscript. F. R. undertook data, interpreted data analysis and critically reviewed the manuscript. C. N. S. and J. L. V. contributed to the drafting and critical revision of the manuscript. M. R. V. designed and conducted the study, formulated the research question, interpreted the data and the statistical analysis and critically reviewed the article.
None of the authors has any conflicts of interest to declare.










