Introduction
Respiratory and gastrointestinal infections in school-age children are frequent morbidities that result in school absenteeism and parental absenteeism from work [Reference Neuzil, Hohlbein and Zhu1]. The risk factors for infection-related morbidity in this age group are understudied.
We have previously reported that plasma 25-hydroxy vitamin D (25(OH)D) is inversely associated with the incidence of gastrointestinal and ear infections in school-age children [Reference Thornton2]. As much as 88% of circulating 25(OH)D is transported by vitamin D-binding protein (DBP) [Reference Bikle3], whose synthesis is largely determined by genetic factors [Reference Lauridsen, Vestergaard and Nexo4]. In addition to its vitamin D-transporting role, DBP may exert a direct modulatory effect on the innate immune response. DBP enhances C5a-induced chemotaxis of neutrophils to an infected area [Reference Trujillo5, Reference Kew, Fisher and Webster6]. Further, upon conversion to DBP macrophage-activating factor (DBP-maf), DBP can activate macrophages that release neutrophil chemo-attractants [Reference Wood7] and engulf antigens [Reference Mohamad8]. DBP also binds extracellular actin to prevent it from polymerizing [Reference Harper9]. These functions may be genotype-dependent, since DBP polymorphisms have been associated with infection-related outcomes, such as rheumatic fever, in children [Reference Bahr10]. However, results from studies of the associations between DBP polymorphisms and other infections, including tuberculosis, are inconsistent [Reference Martineau11–Reference Ralph14]. It is unknown whether DBP concentration may be related to infection-related morbidity in school-age children.
We conducted a prospective study to investigate the associations between DBP and infectious morbidity among school-age children in Bogotá, Colombia. We hypothesised that children with lower DBP concentrations were at increased risk of gastrointestinal and respiratory morbidity. Because DBP might play a role in regulating 25(OH)D concentration and bioavailability [Reference Lauridsen15–Reference Braithwaite18], we also examined whether any associations between DBP and morbidity could be mediated through 25(OH)D.
Methods
Study design and population
We conducted a longitudinal study in the context of the Bogotá School Children Cohort, an investigation of nutrition and health among school-age children in Bogotá, Colombia. Details on the cohort design have been previously reported [Reference Arsenault19]. In brief, in February 2006, we recruited 3202 randomly selected children aged 5–12 years who were enrolled in public primary schools. Since most of the children in the public school system in Bogotá are from low- and middle-income socio-economic backgrounds, the sample is representative of these groups. The parents or primary caregivers of all children gave written informed consent prior to enrolment into the study. Children gave written assent to participate. The study protocol was approved by the Ethics Committee of the National University of Colombia Medical School. The Institutional Review Board at the University of Michigan approved the use of data and samples from the study.
Baseline information
At the time of enrolment, we elicited information from parents on socio-demographic characteristics of the children and their families with the use of a self-administered questionnaire. The survey included questions on maternal age, education level, parity, height and weight. Questions on household characteristics pertained to the level of food insecurity and socio-economic status (SES). Food insecurity was measured according to a scale adapted from the Spanish-language version of the USDA Household Food Security Survey Module [Reference Harrison20]. SES was determined per the city government's classification for tax and planning purposes. The questionnaire also inquired about children's health habits, including usual weekly time spent playing outdoors.
During the weeks following enrolment, trained research assistants performed anthropometric measurements on the children during scheduled school visits. Height was measured without shoes to the nearest 1 mm with wall-mounted Seca 202 stadiometers (Seca, Hanover, MD, USA) and weight was measured in light clothing to the nearest 0.1 kg with Tanita HS301 electronic scales (Tanita, Arlington Heights, IL, USA). Height and weight were also measured among children's mothers if they were present at schools. At these visits, research assistants obtained fasting blood samples through antecubital venipuncture in 88% of children. One aliquot was collected in an EDTA-coated tube for separation of plasma. The samples were protected from sunlight and transported in refrigerated coolers on the day of collection to the Colombian National Institute of Health where they were cryopreserved at −80 °C. Samples were collected from all children during the same season, in February 2006.
Follow-up
During the academic year following enrolment, parents or primary caregivers recorded daily information on the incidence of infectious morbidity symptoms with the use of a pictorial diary. The diaries consisted of drawings that depict children experiencing vomiting, diarrhoea, fever, cough and earache or ear discharge. Caregivers were instructed to mark each day the child had these symptoms or visited a doctor. The pictorial diaries were distributed and returned weekly. Pictorial diaries are an adequate method to capture the incidence of morbidity in low- and middle-income countries [Reference Goldman, Vaughan and Pebley21, Reference Wright22] since their use does not require a high level of education.
Laboratory methods
After separation of plasma, samples were transported frozen to the USA for analyses. Plasma DBP and 25(OH)D were quantified in a random subset of 540 children. DBP was determined using a Quantikine ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) with a monoclonal antibody specific to DBP at the University of Michigan Center for Chemical Genomics (Ann Arbor, MI, USA). All samples were tested in duplicate and the mean of the two replicates was used for analyses. The mean coefficient of variation (CV) for replicate measures was 13%. The 25(OH)D was measured at the Clinical and Epidemiologic Research Laboratory of Children's Hospital Boston (Boston, MA, USA) by an enzyme immunoassay (Immunodiagnostic Systems Inc., Fountain Hills, AZ, USA) with a competitive binding technique. The sensitivity of the assay was 5 nmol 25(OH)D/l. The intraclass CV was 5.3–6.7%, whereas the interclass CV was 4.6–8.7%. All samples were analysed in duplicate.
Data analysis
The primary outcomes of interest were rates of gastrointestinal and respiratory morbidity. We combined symptoms reported on the same day to represent these morbidities; they included diarrhoea with vomiting, cough with fever and earache or ear discharge with fever. These symptoms have been related to clinically diagnosed episodes of gastrointestinal and respiratory infections [Reference de Wit23–Reference Carlin25]. We also considered days of doctor visits. Rates were calculated as the number of days with each symptom combination divided by the number of days the child was under observation.
The main exposure of interest was plasma concentration of DBP. DBP concentration was categorised into quartiles since there are no conventionally accepted cut-points. To identify potential confounders of the association between DBP and morbidity, we first compared the distribution of DBP across levels of baseline child, maternal and household characteristics. Children's height- and body mass index (BMI)-for-age Z scores were estimated according to the World Health Organization growth reference for children and adolescents [Reference de Onis26]. Children's 25(OH)D serostatus was categorised as <50, 50 to <75 or ⩾75 nmol/l according to cut-points recommended by the United States Endocrine Society [Reference Holick27]. Maternal BMI was calculated with measured height and weight in 45% of mothers and from self-reported data in the rest. Household food insecurity was categorised as none, mild, moderate or severe according to the number of affirmative responses to the food insecurity module.
Next, we compared the distributions of days with each symptom combination across quartiles of DBP by estimating the incidence rates and rate ratios (IRR) with 95% confidence intervals (CI) with the use of generalised estimating equations with the Poisson distribution, the log-link function and robust estimates of the variance. Tests for linear trend were conducted by introducing a variable representing median DBP values for each quartile into the model as a continuous predictor. When the associations did not follow a linear pattern, we compared the rate between DBP concentrations at or above quartile 1. In multivariable analysis, we estimated adjusted IRRs and 95% CI from models that included as covariates the correlates of DBP and known independent predictors of morbidity in this population [Reference Thornton2, Reference Thornton28]. We deliberately avoided adjustment for 25(OH)D because DBP concentration may influence 25(OH)D concentration [Reference Lauridsen15, Reference Fu16, Reference Braithwaite18].
We also assessed whether an association between DBP and morbidity was mediated by 25(OH)D under the assumptions of a counterfactual frame [Reference Valeri and Vanderweele29]. For the morbidity outcomes associated with DBP concentration, we estimated the direct associations of DBP (exposure) on morbidity (outcome) independent of 25(OH)D (mediator), the indirect association of DBP mediated through 25(OH)D and the proportion of the total DBP association mediated through 25(OH)D using Valeri and Vanderweele's formulas [Reference Valeri and Vanderweele29]. This method assumes no unmeasured confounding in the exposure–outcome, mediator–outcome and exposure–mediator relations, and no effect of the exposure on confounders of the mediator–outcome relation. DBP concentration was categorised dichotomously (Q1 vs. Q2, 3 or 4) and 25(OH)D was defined as <50 or ⩾50 nmol/l. The exposure–mediator and exposure–outcome relations were modelled with logistic and Poisson regressions, respectively, and adjusted for child's age, sex, stunting and household SES. Mediation analyses were conducted with the use of the %mediation macro [Reference Valeri and Vanderweele29] for the Statistical Analysis System (SAS) software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Mean ± s.d. age of children at recruitment was 8.8 ± 1.6 years; 52% of children were girls. Mean ± s.d. plasma DBP concentration was 2650 ± 1145 nmol/l. Mean ± s.d. plasma 25(OH)D was 73.6 ± 22.8 nmol/l; 9.4% of children had 25(OH)D < 50 nmol/l whereas no child had concentrations <30 nmol/l. Children contributed a total 72 106 days of observation with a median (interquartile range) of 147 (91, 182) days per child; the distribution of total child-days did not vary significantly by quartile of DBP. Overall rates (days per child-year) of diarrhoea with vomiting, cough with fever, earache or ear discharge with fever, and doctor visits were, respectively, 0.79, 2.89, 0.42 and 2.13. Correlations between event rates ranged from 0.2 between earache or ear discharge with fever and doctor visits to 0.33 between cough with fever and earache or ear discharge with fever (Supplementary Table S1). At baseline, DBP concentration was positively associated with stunting (height-for-age Z score <−2) and plasma 25(OH)D concentration (Table 1).
Table 1. Plasma concentration of vitamin D-binding protein (DBP) according to child, maternal and household characteristics in school-age children from Bogotá, Colombia
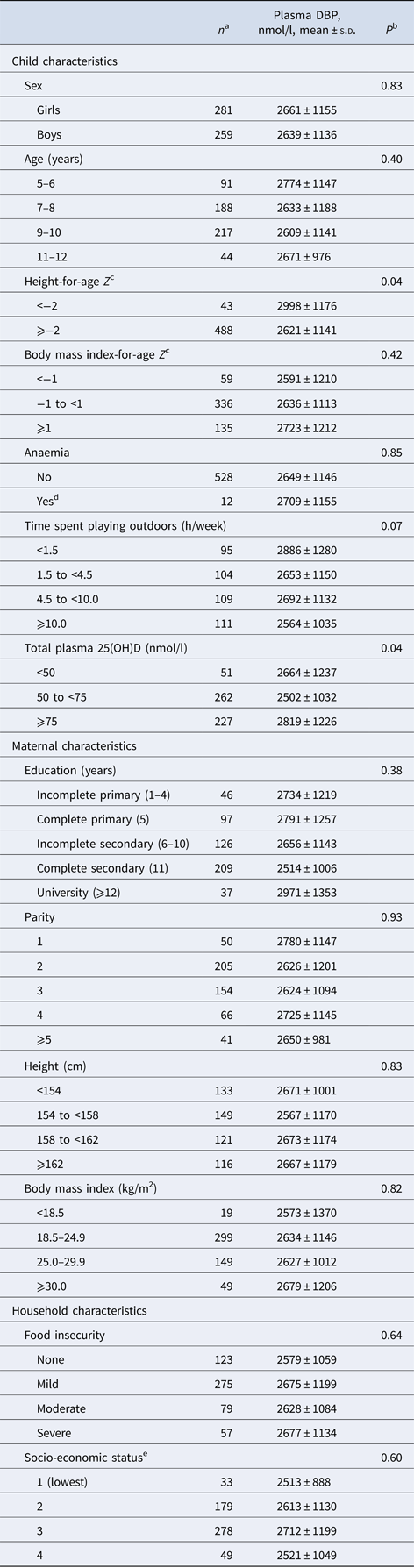
a Sums may be <540 due to missing values in covariates.
b For child's sex, and anaemia Wald test. For all other characteristics, test for linear trend when a variable representing ordinal categories of the predictor was introduced as a continuous covariate into a linear regression model with DBP concentration as the continuous outcome. Empirical estimates of the variance and an exchangeable correlation structure were specified in all models.
c According to the World Health Organization growth reference for children and adolescents (27).
d Haemoglobin under the altitude-adjusted cut-point (12.7 g/dl).
e According to the city government's classification of households for public service fees.
DBP concentration was inversely associated with the incidence of diarrhoea with vomiting and earache or ear discharge with fever in a non-linear manner (Table 2). In multivariable analysis, the rates of diarrhoea with vomiting among children in quartiles 2–4 of DBP concentration were 52% lower compared with the rates among children in quartile 1 after adjustment for child's sex, age, stunting and household SES (adjusted IRR = 0.48; 95% CI 0.25–0.92; P = 0.03). Rates of earache or ear discharge with fever in children with DBP concentration in quartiles 2–4 were 71% lower than those of children in the first quartile (adjusted IRR = 0.29; 95% CI 0.12–0.71; P = 0.006) (Table 2). DBP concentration was inversely related to the incidence of cough with fever and to doctor visits, but these associations were not statistically significant.
Table 2. Morbidity and doctor visits according to quartiles of plasma vitamin D-binding protein (DBP) concentration in school-age children from Bogotá, Colombia
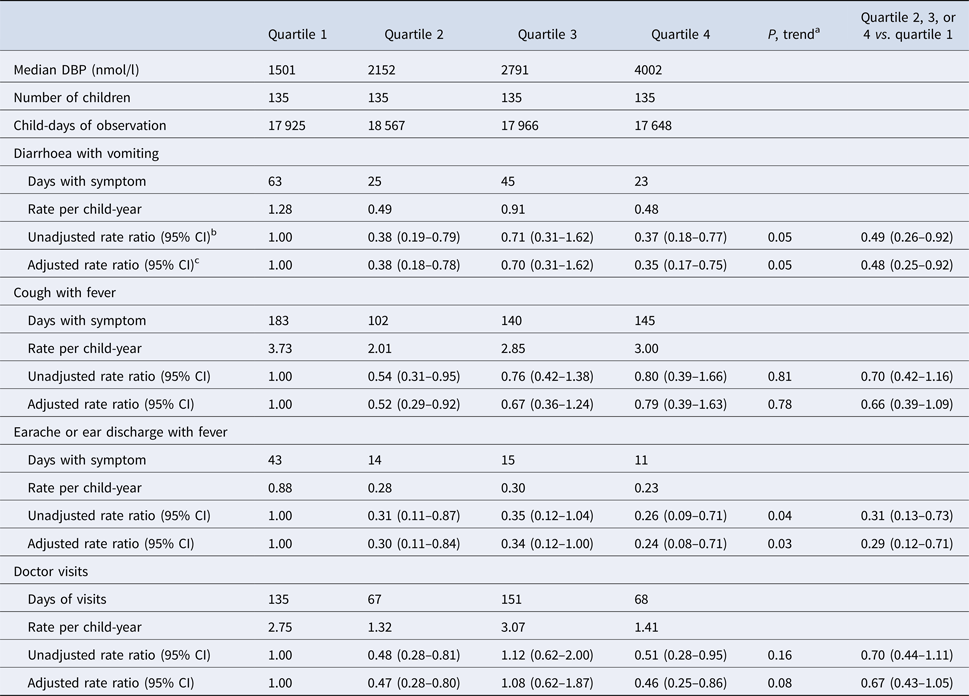
a Test for linear trend when a variable representing the median value of DBP quartiles was introduced as a continuous covariate into a generalised estimating equations (GEE) model with the Poisson distribution in which days with each combination of symptoms or doctor visits were the outcome. Empirical estimates of the variance and an exchangeable correlation structure were specified in all models.
b From GEE with the Poisson distribution and the log-link with days with each combination of symptoms as the outcome, indicator variables for quartiles 2, 3 and 4 of DBP as predictors, and total days under observation as the offset variable.
c Adjusted for sex (dichotomous), baseline age (continuous), height-for-age <−2 (dichotomous) and household socio-economic status (categorical).
The associations between DBP concentration and diarrhoea with vomiting or earache or ear discharge with fever were not mediated through 25(OH)D (% mediated = 0.6% and 0.6%, respectively) (Table 3). There was not a significant interaction between DBP and 25(OH)D on these outcomes.
Table 3. Mediation by plasma 25-hydroxy vitamin D (25(OH)D) of the association between vitamin D-binding protein (DBP) and morbidity
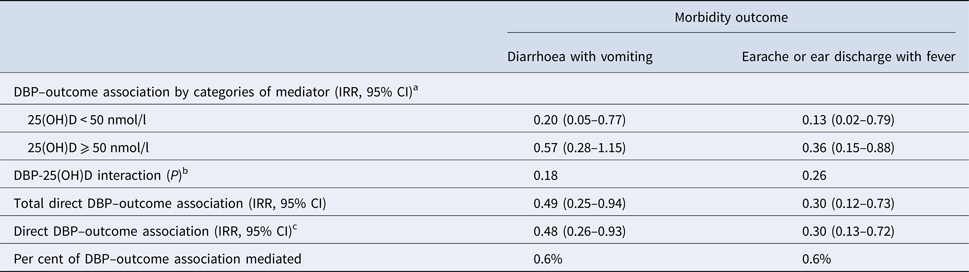
a From Poisson regression adjusted for sex (dichotomous), baseline age (continuous), height-for-age Z < −2 (dichotomous) and household socio-economic status (categorical).
b Wald test for an interaction term between DBP >quartile 1 and 25(OH)D < 50 nmol/l.
c Estimated at mediator level <50 nmol/l.
Discussion
In this prospective study of school-age children, plasma DBP concentration was inversely associated with the rates of diarrhoea with vomiting and earache or ear discharge with fever, independent of child's sex, age, stunting and household SES. These associations were not mediated through 25(OH)D.
The association of lower DBP concentration with higher rates of diarrhoea with vomiting could have resulted from higher incidence and/or longer duration of gastrointestinal infections. Norovirus infection is a frequent cause of gastroenteritis in school-age children and could have underlain many of the cases observed. Norovirus infects macrophages, dendritic cells and B cells [Reference Karst and Tibbetts30]. It is possible that a macrophage-differentiation effect of DBP-maf be related to an inverse association between circulating DBP and viral gastroenteritis. Diarrhoea with vomiting could also result from bacterial infections, including Salmonella and Shigella [Reference Wiegering31, Reference Karacan32]. Actin polymerisation is an important mechanism in the control of Salmonella infection [Reference Man33] and DBP binds to actin with a very high affinity [Reference Verboven34]; thus, competitive binding of actin or interference with its polymerisation might influence the pathogen's ability to infect epithelial cells. Shigella downregulates mucosal antimicrobial peptides with phagocytic and chemoattractant properties [Reference Gudmundsson35]. The macrophage-activating and chemotaxis-enhancing functions of DBP might counteract the downregulation of the antimicrobial peptides. Thus, children with lower DBP concentrations may be more susceptible to infection by these microorganisms or to develop more severe symptoms related to these infections.
DBP concentration was also strongly inversely associated with earache or ear discharge with fever, which are symptoms of otitis media. In children, otitis media is most often caused by the bacterial pathogens Haemophilus influenzae and Streptococcus pneumoniae [Reference Bluestone, Stephenson and Martin36, Reference Rovers37]. Non-typable H. influenzae and S. pneumoniae colonise the nasopharynx in a high percentage of children [Reference St Geme38, Reference Bergenfelz and Hakansson39] and viral upper respiratory infections can induce the onset of acute otitis media in children with existing bacterial colonisation [Reference Chonmaitree40]. DBP may be associated with lower incidence of viral upper respiratory infections through recruitment of neutrophils and enhanced phagocytosis [Reference Trujillo5, Reference Mohamad8], thus lowering the incidence of otitis media.
The associations of DBP with gastrointestinal or ear infections were non-linear; there was some evidence for a ‘threshold effect’ in that the incidence in DBP quartile 2, 3 or 4 was higher than that in quartile 1. Although the biologic mechanisms underlying a potential threshold effect are unknown, similar non-linear associations have been reported between DBP and other outcomes in adults, such as pancreatic cancer [Reference Weinstein41].
We have previously reported that plasma 25(OH)D was inversely related to diarrhoea with vomiting and earache or ear discharge with fever in this population [Reference Thornton2]. In the present study, DBP and 25(OH)D concentrations were positively associated at the time of recruitment. DBP may regulate circulating 25(OH)D concentration and bioavailability; thus, it may be possible that the previously observed associations between 25(OH)D and these outcomes be due to DBP. It was also plausible that the association of DBP with morbidity be mediated through 25(OH)D. Results from mediation analyses, however, indicated that the DBP–morbidity associations were largely independent of 25(OH)D. Therefore, DBP and 25(OH)D might have independent effects on morbidity outcomes. We noted that stunting, an indicator of chronic undernutrition and past infection burden, was related to low DBP at baseline. Even though stunting was not related to infectious morbidity in this population [Reference Thornton28], its association with DBP may suggest that DBP could be a biomarker of general health that predicts predisposition to infection, even if it is not causally related to it.
This study has several strengths. First, its longitudinal design minimises the possibility of reverse causation bias. Second, prospective collection of morbidity precludes misclassification of outcomes. Third, we had the possibility to control for important potential confounders of the relation between DBP and morbidity. Finally, we had an opportunity to evaluate whether the association between DBP and morbidity was mediated through 25(OH)D using state-of-the-art analytic techniques.
Some limitations are also noteworthy. We used a monoclonal assay to quantify DBP. With this assay, 85% of the variation in DBP concentration is explained by genotype [Reference Denburg42]. There are three major DBP alleles: Gc1F, Gc1s and Gc2. If some alleles are associated with the incidence of infectious morbidity, our results could be confounded by genetics. For example, the Gc2 polymorphism of DBP does not convert into DBP-maf effectively [Reference Wood7]. In Hispanic populations, 35–38% have the Gc2 polymorphism [Reference Carpenter17, Reference Navas-Nazario43]. The use of a polyclonal DBP assay, which was unavailable at the time we conducted the study, could overcome this problem. Another limitation is that a one-time measurement of DBP or 25(OH)D concentrations may not represent long-term exposure status. However, repeated measures of DBP concentration in adults indicate that concentrations may be stable over time [Reference Ralph14]. Similarly, correlation of repeated measures of 25(OH)D concentration over long periods is high, suggesting that a single measurement may represent long-term exposure [Reference Jorde44]. Because outcomes were defined based on the total number of days with symptoms, we were unable to differentiate the incidence from duration of the episodes; this differentiation could have informed underlying mechanisms. Finally, we lacked pathogen-specific information during infectious morbidity episodes, and this limits the ability to postulate specific mechanisms underlying a potential effect of DBP on infections.
In conclusion, plasma DBP concentration is inversely associated with the symptoms of gastrointestinal and ear infections in school-age children, independent of 25(OH)D serostatus. These results warrant confirmation in other populations. Whether DBP concentrations can be modified through intervention remains to be determined.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818002066.
Financial support
This work was supported by the ASISA Research Fund at the University of Michigan.
Conflict of interest
None.








