Introduction
Both environmental and genetic factors are associated with an increased risk of developing psychotic disorders (van Os et al., Reference van Os, Kenis and Rutten2010). The relationships between these factors have long been discussed, and the hypothesis of genes x environment (GxE) interactions was suggested several decades ago (Strahilevitz, Reference Strahilevitz1974; Murray et al., Reference Murray, Reveley and McGuffin1986; Schulsinger et al., Reference Schulsinger, Parnas, Mednick, Teasdale and Schulsinger1987). Such interaction can be defined as a genetic modulation of the sensitivity to environmental factors and/or environmental control of the gene expression (Kendler and Eaves, Reference Kendler and Eaves1986). Numerous studies supported this hypothesis (Collip et al., Reference Collip, Myin-Germeys, Wichers, Jacobs, Derom, Thiery, Lataster, Simons, Delespaul, Marcelis, van Os and van Winkel2013; Frydecka et al., Reference Frydecka, Kotowicz, Gawęda, Prochwicz, Kłosowska, Rymaszewska, Samochowiec, Samochowiec, Podwalski, Pawlak-Adamska, Szmida, Cechnicki and Misiak2020; Pries et al., Reference Pries, Ferro, van Os, Delespaul, Kenis, Lin, Luykx, Richards, Akdede, Binbay, Altınyazar, Yalınçetin, Gümüş-Akay, Cihan, Soygür, Ulaş, Cankurtaran, Kaymak, Mihaljevic, Petrovic, Mirjanic, Bernardo, Mezquida, Amoretti, Bobes, Saiz, García-Portilla, Sanjuan, Aguilar, Santos, Jiménez-López, Arrojo, Carracedo, López, González-Peñas, Parellada, Maric, Atbaşoğlu, Ucok, Alptekin, Saka, Arango, O'Donovan, Tosato, Rutten and Gülöksüz2020a), and particularly one from Caspi et al. (Reference Caspi, Moffitt, Cannon, McClay, Murray, Harrington, Taylor, Arseneault, Williams, Braithwaite, Poulton and Craig2005), in which a significant interaction between cannabis use in adolescence and the genetic variant Val158Met in the Catechol-O-Methyltransferase (COMT, which metabolises dopamine) gene was found. In this study, in comparison to Val/Val genotype, Met/Met and Met/Val genotypes had a protective effect against the risk of psychotic symptoms and disorders among cannabis users (in the group of subjects without cannabis use, the rates of psychotic symptoms and disorders were similar according to the different genotypes). Of note, although discrepant results have been reported in replication studies (Henquet et al., Reference Henquet, Rosa, Krabbendam, Papiol, Faňanás, Drukker, Ramaekers and van Os2006; Zammit et al., Reference Zammit, Spurlock, Williams, Norton, Williams, O'Donovan and Owen2007), a meta-analysis confirmed the small protective effect of the Val/Met heterozygous genotype [pooled OR = 0.947 95% CI (0.904–0.993), p = 0.023] (Costas et al., Reference Costas, Sanjuán, Ramos-Ríos, Paz, Agra, Ivorra, Páramo, Brenlla and Arrojo2011). This meta-analysis, that did not take account of the cannabis use, hypothesised that both too high and too low levels of dopamine could be risk factors. Study of GxE interactions is difficult due to the need for large cohorts with well-characterised genetic and environmental data.
To deal with these difficulties, the study of subclinical psychosis in the general population, that can be defined as psychotic symptoms in subjects who do not meet criteria for psychotic disorders, is convenient (Verdoux and van Os, Reference Verdoux and van Os2002; McGrath et al., Reference McGrath, Saha, Al-Hamzawi, Alonso, Bromet, Bruffaerts, Caldas-de-Almeida, Chiu, de Jonge, Fayyad, Florescu, Gureje, Haro, Hu, Kovess-Masfety, Lepine, Lim, Mora, Navarro-Mateu, Ochoa, Sampson, Scott, Viana and Kessler2015), especially in accordance to the aetiological psychotic continuum hypothesis. According to this hypothesis, subclinical psychosis has a similar origin/aetiology as psychotic disorders (Linscott and van Os, Reference Linscott and van Os2013; van Os, Reference van Os2014; Pignon et al., Reference Pignon, Schürhoff, Szöke, Geoffroy, Jardri, Roelandt, Rolland, Thomas, Vaiva and Amad2018; Pries et al., Reference Pries, Gülöksüz, Ten Have, de Graaf, van Dorsselaer, Gunther, Rauschenberg, Reininghaus, Radhakrishnan, Bak, Rutten and van Os2018). Thus, studying genetic or environmental risk factors associated with subclinical psychosis may provide insights into the aetiology of psychosis and partly reduce the potential interference of reverse causation, i.e. factors are associated with or caused by the clinical disorders themselves [e.g. hospitalisations, stigma, substance use disorders or social drift after onset (Zipursky, Reference Zipursky2014; Sariaslan et al., Reference Sariaslan, Fazel, D'Onofrio, Långström, Larsson, Bergen, Kuja-Halkola and Lichtenstein2016; Pignon et al., Reference Pignon, Eaton, Schürhoff, Szöke, McGorry and O'Donoghue2019a)]. Furthermore, in line with the continuum theory, subclinical psychosis can be characterised by continuous variables, improving statistical power, which is a key issue in GxE interaction studies.
Psychotic disorders are characterised by a polygenic architecture, with thousands of common genetic variants with small effect sizes, and a few rare variants with large effect sizes (Smeland et al., Reference Smeland, Frei, Dale and Andreassen2020). The genome-wide effects of disease-associated common genetic variants can be summarised in a polygenic risk score (PRS) (Anderson et al., Reference Anderson, Shade, DiBlasi, Shabalin and Docherty2019), which offers new opportunities to characterise the complex genetic aetiology of psychotic disorders. In subjects included through the EUropean network of national schizophrenia networks studying Gene-Environment Interactions (EU-GEI), the PRS for schizophrenia (PRS-SZ) explained between 7 and 9% of the variance of the case-control status (Di Forti et al., Reference Di Forti, Wu-Choi, Quattrone, Richards, Freeman, Tripoli, Gayer-Anderson, Rodriguez, Jongsma, Ferraro, Cascia, Tosato, Tarricone, Berardi, Szoke, Arango, Bobes, Sanjuan, Santos, Arrojo, Velthorst, Bernardo, Ben, Selten, Jones, Kirkbride, Rutten, de Haan, van Os, Lynskey, Morgan, Vassos, O'Donovan, Lewis, Sham and Murray2019b; Tripoli et al., Reference Tripoli, Quattrone, Ferraro, Gayer-Anderson, Rodriguez, La Cascia, La Barbera, Sartorio, Seminerio, Tarricone, Berardi, Szöke, Arango, Tortelli, Llorca, de Haan, Velthorst, Bobes, Bernardo, Sanjuán, Santos, Arrojo, Del-Ben, Menezes, Selten, Jones, Jongsma, Kirkbride, Lasalvia, Tosato, Richards, O'Donovan, Rutten, van Os, Morgan, Sham, Murray, Murray and Di Forti2020), consistently with other studies (Vassos et al., Reference Vassos, Di Forti, Coleman, Iyegbe, Prata, Euesden, O'Reilly, Curtis, Kolliakou, Patel, Newhouse, Traylor, Ajnakina, Mondelli, Marques, Gardner-Sood, Aitchison, Powell, Atakan, Greenwood, Smith, Ismail, Pariante, Gaughran, Dazzan, Markus, David, Lewis, Murray and Breen2017). Of note, among patients with psychotic disorders, the PRS-SZ is also associated with antipsychotic treatment response, the level of quality of life, or, in the general population, to the intelligence quotient (IQ), and the risk of attention-deficit/hyperactivity disorder (ADHD) (Mistry et al., Reference Mistry, Harrison, Smith, Escott-Price and Zammit2018; Legge et al., Reference Legge, Jones, Kendall, Pardiñas, Menzies, Bracher-Smith, Escott-Price, Rees, Davis, Hotopf, Savage, Posthuma, Holmans, Kirov, Owen, O'Donovan, Zammit and Walters2019; Zhang et al., Reference Zhang, Robinson, Yu, Gallego, Fleischhacker, Kahn, Crespo-Facorro, Vazquez-Bourgon, Kane, Malhotra and Lencz2019; Pries et al., Reference Pries, van Os, Ten Have, de Graaf, van Dorsselaer, Bak, Lin, van Eijk, Kenis, Richards, O'Donovan, Luykx, Rutten and Gülöksüz2020c).
Studies of associations between subclinical psychosis and the PRS-SZ have produced contradictory results (Zammit et al., Reference Zammit, Hamshere, Dwyer, Georgiva, Timpson, Moskvina, Richards, Evans, Lewis, Jones, Owen and O'Donovan2014; Mistry et al., Reference Mistry, Harrison, Smith, Escott-Price and Zammit2018; Legge et al., Reference Legge, Jones, Kendall, Pardiñas, Menzies, Bracher-Smith, Escott-Price, Rees, Davis, Hotopf, Savage, Posthuma, Holmans, Kirov, Owen, O'Donovan, Zammit and Walters2019; Nenadić et al., Reference Nenadić, Meller, Schmitt, Stein, Brosch, Mosebach, Ettinger, Grant, Meinert, Opel, Lemke, Fingas, Förster, Hahn, Jansen, Andlauer, Forstner, Heilmann-Heimbach, Hall, Awasthi, Ripke, Witt, Rietschel, Müller-Myhsok, Nöthen, Dannlowski, Krug, Streit and Kircher2020), and further studies are needed. Moreover, to date, four studies have investigated the role of GxE interaction on subclinical psychosis using PRS-SZ. Two studies assessed the interaction between PRS-SZ and childhood trauma, but only one reported a significant interaction (Pries et al., Reference Pries, Klingenberg, Menne-Lothmann, Decoster, van Winkel, Collip, Delespaul, Hert, Derom, Thiery, Jacobs, Wichers, Cinar, Lin, Luykx, Rutten, van Os and Gülöksüz2020b), whereas the other showed an independent (additive) effects of the PRS-SZ and the trauma without significant interaction (Trotta et al., Reference Trotta, Iyegbe, Forti, Sham, Campbell, Cherny, Mondelli, Aitchison, Murray, Vassos and Fisher2016). A recent study assessing the associations between momentary stress and subclinical psychotic symptoms showed that higher levels of PRS-SZ were associated with a higher intensity of symptoms after a momentary stress among controls (Schick et al., Reference Schick, van Winkel, Lin, Luykx, de Zwarte, van Eijk, Myin-Germeys and Reininghaus2022). In the fourth study, the authors assessed the interaction between PRS-SZ and smoking status, but did not show significant association (García-González et al., Reference García-González, Ramírez, Howard, Brennan, Munroe and Keers2020).
In addition to increasing the risk for psychosis by GxE interactions, the PRS-SZ has also been associated with a greater risk of exposure to environmental risk factors for psychosis (Pingault et al., Reference Pingault, O'Reilly, Schoeler, Ploubidis, Rijsdijk and Dudbridge2018). For instance, several studies have reported associations between the PRS-SZ and cannabis use (Gage et al., Reference Gage, Jones, Burgess, Bowden, Smith, Zammit and Munafò2017; Pasman et al., Reference Pasman, Verweij, Gerring, Stringer, Sanchez-Roige, Treur, Abdellaoui, Nivard, Baselmans, Ong, Ip, van der Zee, Bartels, Day, Fontanillas, Elson, de Wit, Davis, MacKillop, Derringer, Branje, Hartman, Heath, van Lier, Madden, Mägi, Meeus, Montgomery, Oldehinkel, Pausova, Ramos-Quiroga, Paus, Ribases, Kaprio, Boks, Bell, Spector, Gelernter, Boomsma, Martin, MacGregor, Perry, Palmer, Posthuma, Munafò, Gillespie, Derks and Vink2018) or between the PRS-SZ and urbanicity (Colodro-Conde et al., Reference Colodro-Conde, Couvy-Duchesne, Whitfield, Streit, Gordon, Kemper, Yengo, Zheng, Trzaskowski, de Zeeuw, Nivard, Das, Neale, MacGregor, Olsen, Whiteman, Boomsma, Yang, Rietschel, McGrath, Medland and Martin2018; Paksarian et al., Reference Paksarian, Trabjerg, Merikangas, Mors, Børglum, Hougaard, McGrath, Pedersen, Mortensen and Agerbo2018; Maxwell et al., Reference Maxwell, Coleman, Breen and Vassos2021) or the level of neighbourhood deprivation and social fragmentation at birth (Solmi et al., Reference Solmi, Lewis, Zammit and Kirkbride2020), challenging the traditional gene v. environment dichotomy. However, these observations could not explain the strength of the associations between cannabis use or urbanicity and the risk of psychotic disorders (Vassos et al., Reference Vassos, Pedersen, Murray, Collier and Lewis2012; Di Forti et al., Reference Di Forti, Quattrone, Freeman, Tripoli, Gayer-Anderson, Quigley, Rodriguez, Jongsma, Ferraro, La Cascia, La Barbera, Tarricone, Berardi, Szöke, Arango, Tortelli, Velthorst, Bernardo, Del-Ben, Menezes, Selten, Jones, Kirkbride, Rutten, de Haan, Sham, van Os, Lewis, Lynskey, Morgan, Murray, Amoretti, Arrojo, Baudin, Beards, Bernardo, Bobes, Bonetto, Cabrera, Carracedo, Charpeaud, Costas, Cristofalo, Cuadrado, Díaz-Caneja, Ferchiou, Franke, Frijda, García Bernardo, Garcia-Portilla, González, Hubbard, Jamain, Jiménez-López, Leboyer, López Montoya, Lorente-Rovira, Marcelino Loureiro, Marrazzo, Martínez, Matteis, Messchaart, Moltó, Nacher, Olmeda, Parellada, González Peñas, Pignon, Rapado, Richard, Rodríguez Solano, Roldán Díaz, Ruggeri, Sáiz, Sánchez, Sanjuán, Sartorio, Schürhoff, Seminerio, Shuhama, Sideli, Stilo, Termorshuizen, Tosato, Tronche, van Dam and van der Ven2019a). To the best of our knowledge, the genetic-environment (G-E) associations between PRS-SZ and psychosocial stressors have not been studied to date.
In a former study on population-based controls from the EU-GEI work package 2 (WP2) (Pignon et al., Reference Pignon, Lajnef, Kirkbride, Peyre, Ferchiou, Richard, Baudin, Tosato, Jongsma, de Haan, Tarricone, Bernardo, Velthorst, Braca, Arango, Arrojo, Bobes, Del-Ben, Di Forti, Gayer-Anderson, Jones, La Cascia, Lasalvia, Menezes, Quattrone, Sanjuán, Selten, Tortelli, Llorca, van Os, Rutten, Murray, Morgan, Leboyer, Szöke and Schürhoff2021), we showed that psychosocial stressors, i.e. childhood trauma, stressful life-events, self-reported discrimination experiences and low social capital, had independent effects on subclinical psychosis dimensions, without significant environment x environment (ExE) interactions. In the current study, we aimed to study the relationships between these psychosocial stressors, the PRS-SZ, and three dimensions of subclinical psychosis (positive, negative, depressive), looking for GxE interaction. Furthermore, we aimed to study the association between psychosocial stressors and the PRS-SZ, looking for G-E associations.
Methods
EU-GEI WP2 study
Clinical, environmental and genetic data have been collected through the EU-GEI WP2 (named ‘Functional Enviromics’), a multicentre case-sibling-control study of genetic and environmental determinants of the occurrence and severity of psychotic disorders. Population-based controls were recruited across 6 countries: Brazil, France, Italy, the Netherlands, Spain, and the United Kingdom. Inclusion criteria were: age 18–64, living in the catchment areas, no evidence of current or past psychotic disorders. These controls were recruited using a mixture of random and quota sampling to ensure that they were broadly representative of the at-risk populations on predefined variables (age, sex, and migration) (Gayer-Anderson et al., Reference Gayer-Anderson, Jongsma, Di Forti, Quattrone, Velthorst, de Haan, Selten, Szöke, Llorca, Tortelli, Arango, Bobes, Bernardo, Sanjuán, Santos, Arrojo, Parellada, Tarricone, Berardi, Ruggeri, Lasalvia, Ferraro, La Cascia, La Barbera, Menezes, Del-Ben, Hubbard, Beards, Reininghaus, Tripoli, Stilo, Parellada, Roldán, López, Matteis, Rapado, González, Martínez, Cuadrado, Solano, Carracedo, Costas, Bernardo, Sánchez, Olmeda, Cabrera, Lorente-Rovira, Garcia-Portilla, Jiménez-López, Franke, van Dam, Termorshuizen, van der Ven, Messchaart, Leboyer, Schürhoff, Baudin, Ferchiou, Pignon, Jamain, Richard, Charpeaud, Tronche, Frijda, Sideli, Seminerio, Sartorio, Marrazzo, Loureiro, Shuhama, Ruggeri, Tosato, Bonetto, Cristofalo, Rutten, van Os, Jones, Murray, Kirkbride and Morgan2020).
Ethical approval was obtained from local research ethics committees in each country. The EU-GEI Project was funded by the European Community's Seventh Framework Program under grant agreement no. HEALTH-F2-2010-241909.
Subclinical psychosis and psychosocial stressors assessment
The Community Assessment of Psychic Experiences (CAPE) is a 42-item self-report questionnaire that has been developed to assess lifetime subclinical psychotic dimensions in the general population (Stefanis et al., Reference Stefanis, Hanssen, Smirnis, Avramopoulos, Evdokimidis, Stefanis, Verdoux and van Os2002). For each item, 4 answers were possible according to the frequency of their occurrences (from never to nearly always). To construct the dimension scores [positive, negative and depressive (Mark and Toulopoulou, Reference Mark and Toulopoulou2016)], we dichotomised answers of each CAPE item (never v. sometimes or more) and summed the positive answers. The cross-national invariance of the CAPE score in the EU-GEI WP2 samples was previously demonstrated (Pignon et al., Reference Pignon, Peyre, Ferchiou, van Os, Rutten, Murray, Morgan, Leboyer, Schürhoff and Szöke2019b).
Childhood trauma was assessed with a short version of the Childhood Trauma Questionnaire (CTQ), with 25 items assessing five different domains (emotional and physical neglect, emotional, physical and sexual abuse) (Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia, Stokes, Handelsman, Medrano, Desmond and Zule2003). Only the total score was used. Lifetime self-reported discrimination experiences were assessed with a modified version of the 12-item Williams' major experiences of discrimination scale (unfairly fired or not hired because of your ethnicity/sex/weight/etc., unfairly stopped/questioned/physically threatened or abused by the police, etc.) (Williams et al., Reference Williams, Yu, Jackson and Anderson1997; Jongsma et al., Reference Jongsma, Gayer-Anderson, Tarricone, Velthorst, van der Ven, Quattrone, di Forti, Group, Menezes, Del-Ben, Arango, Lasalvia, Berardi, Cascia, Bobes, Bernardo, Sanjuán, Santos, Arrojo, de Haan, Tortelli, Szöke, Murray, Rutten, van Os, Morgan, Jones and Kirkbride2020). Perceived social capital in each participant's immediate neighbourhood was assessed using the Social Environment Assessment Tool (SEAT), a 23-item questionnaire, that was designed to capture four dimensions of social capital: civic disorder (CD), impact of civic disorder (ICD), informal social control (ISC), and social cohesion and trust (SCT) (Sampson et al., Reference Sampson, Raudenbush and Earls1997; Lochner et al., Reference Lochner, Kawachi and Kennedy1999; McCulloch, Reference McCulloch2003; Drukker et al., Reference Drukker, Krabbendam, Driessen and van Os2006). Subjects answer according to a five-point Likert-scale (1: unusual, to 5: very common), and a sum of the weighted scores of the 4 subscales were calculated to obtain the total social capital score (SEAT score = zCD + 0.51 × zICD + 1.6 × zISC + zSCT). Finally, stressful life events were assessed using the List of Threatening Experiences (LTE) which comprises 20 binary items of events usually associated with major stress over the course of the previous 6 months (e.g. serious injury, death of a parent, separation from a partner, financial difficulties) (Brugha et al., Reference Brugha, Bebbington, Tennant and Hurry1985; Motrico et al., Reference Motrico, Moreno-Küstner, de Dios Luna, Torres-González, King, Nazareth, Montón-Franco, Josefa Gilde Gómez-Barragán, Sánchez-Celaya, Ángel Díaz-Barreiros, Vicens, Moreno-Peral and Ángel Bellón2013).
Calculation of a polygenic risk score for schizophrenia (PRS-SZ)
Blood samples of the control sample were genotyped by the Medical Research Council Centre for Neuropsychiatric Genetics and Genomics (Cardiff, United Kingdom) using a custom ‘Illumina HumanCoreExome-24 BeadChip’ genotyping array, covering 570 038 genetic variants. As described elsewhere (Di Forti et al., Reference Di Forti, Wu-Choi, Quattrone, Richards, Freeman, Tripoli, Gayer-Anderson, Rodriguez, Jongsma, Ferraro, Cascia, Tosato, Tarricone, Berardi, Szoke, Arango, Bobes, Sanjuan, Santos, Arrojo, Velthorst, Bernardo, Ben, Selten, Jones, Kirkbride, Rutten, de Haan, van Os, Lynskey, Morgan, Vassos, O'Donovan, Lewis, Sham and Murray2019b), the PRS-SZ were generated using PRSice from the summary results of the Psychiatric Genomics Consortium (PGC), wave 2 (Schizophrenia Working Group of the PGC, 2014). Clumping was performed to obtain SNPs in approximate linkage disequilibrium with an r 2 < 0.25 within a 250 kb window. PRS-SZ were calculated, at p-value thresholds of 0.05, because this threshold best captures liability to the disorder according to the PGC analysis (Schizophrenia Working Group of the PGC, 2014). The sample was restricted to 706 European descendant subjects (due to over-representation of European descendant subjects in the PGC2 training sample used to calculate the PRS-SZ).
Statistical analyses
The G-E association has been assessed by Spearman correlation tests between the 4 psychosocial stressors and the PRS-SZ. Then, linear regression models were used to assess the relationships between the CAPE dimensions scores (positive, negative, depressive), environmental and genetic variables, and to look for GxE interactions. Of note, we consider multiplicative interactions (Rothman et al., Reference Rothman, Greenland and Walker1980; VanderWeele and Knol, Reference VanderWeele and Knol2014).
The different models were adjusted for age, sex, and the 10 first principal components (PCs) of the genetic analyses of the ethnic variance. For each CAPE dimension, thirteen models were tested:
(1) A ‘Genetic model’, with the sole PRS-SZ;
(2) Four ‘Environmental models’ for each of the 4 psychosocial stressors variables: childhood trauma, stressful life-events, self-reported discrimination experiences and low social capital;
(3) Four ‘Independent models’: one for each of the 4 psychosocial stressors variables and the PRS-SZ, without interaction term;
(4) Four ‘Interaction models’: each of the 4 psychosocial stressors variables and the PRS-SZ, with a GxE interaction term.
To compare the fit of the different models (and particularly the Independent and the Interaction models), we compared the explained variances (R 2), and use likelihood ration test (LRT) to assess whether the addition of a factor (E + G v. G, E + G v. E, E + G + E × G v. E + G) improved the fit of the model. To verify that the results were not biased by the imputation of the outcome (CAPE scales), analyses were repeated in a sample without imputation of the CAPE.
Psychosocial variables and PRS-SZ were standardised to Z-scores (i.e. to a mean equal to 0, and a standard-deviation equal to 1). The SEAT (social capital) score was inverted, so that higher scores were associated with lower social capital. Missing data of the CAPE (between 3 and 5.9% according to the different dimensions) and the psychosocial stressors variables (between 0.5 and 20.7%) were imputed with multivariate imputation by chained equations (MICE) in 20 resamples (the country was added to CAPE and psychosocial stressors variables for the imputation). R software version 3.6.0 was used for the statistical analyses.
Results
Description of the data
The 706 European descendant subjects without psychotic disorders included in our study showed a sex ratio close to 1 (53% women) and a mean age of 38.2 (s.d. = 13.4) (% of missing data according to the different countries are available in the online Supplementary Table 3). The scores of subclinical psychosis dimensions and psychosocial stressors scales, and the values of PRS-SZ scores are available in the Table 1 (for non-imputed data, see online Supplementary Table 2).
Table 1. Description of the data: socio-demographic, subclinical psychosis, psychosocial stressors and polygenic risk scores variables

CAPE, Community Assessment of Psychic Experiences’; IQR, interquartile range; PRS-SZ, polygenic risk score for schizophrenia; s.d., standard-deviation.
Correlation between genetic vulnerability and environmental factors
Spearman correlation tests did not suggest any evidence of associations between psychosocial stressors levels and the PRS-SZ (Table 2).
Table 2. Spearman tests between Z-scores of genetic and environmental factors among subjects with complete data (N = 456)
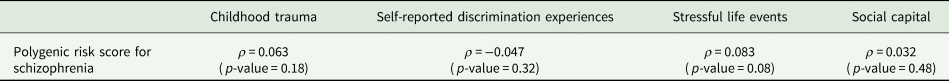
Legend: ρ: Spearman correlation coefficient.
Influence of genetic vulnerability and environmental factors on subclinical psychosis dimensions
For the three subclinical psychosis dimensions that we studied, we first assessed the variance that might be explained by the PRS-SZ (Genetic models, Fig. 1 and Table 3). Only the positive dimension was associated with the PRS-SZ (β = 0.086, p-value = 0.02, with a R 2 = 7.77%).

Fig. 1. Explained variances of the different models.
Table 3. Model comparison of the explained variances of the subclinical psychosis dimensions
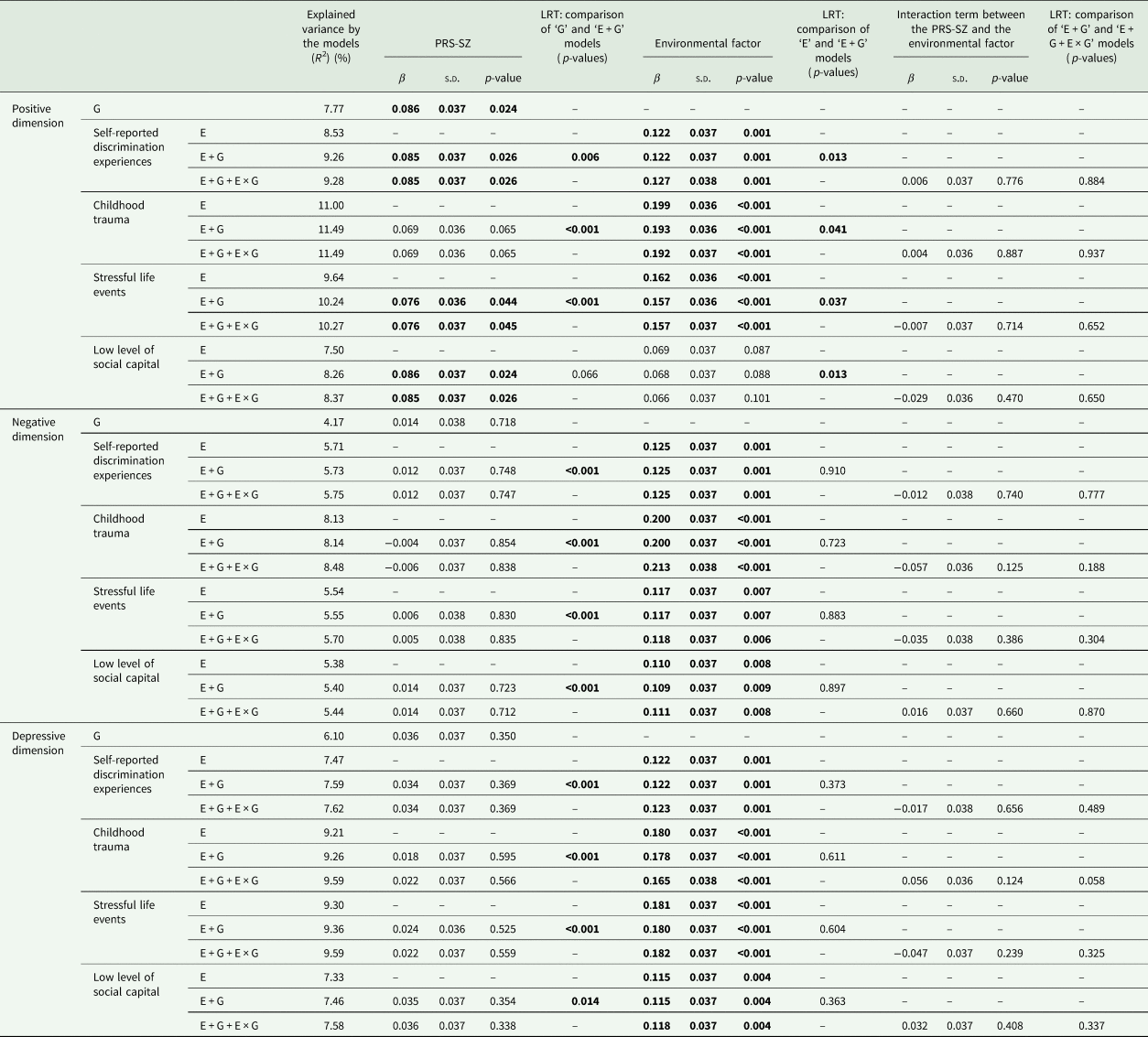
E, Environmental model; E + G, Independent model; E + G + E × G, Interaction model; G, Genetic model; LRT, Likelihood ratio test; PRS-SZ, polygenic risk score for schizophrenia.
The different models were adjusted on age, sex, and the first ten principal components of the ethnicity-based genetic variance.
The significant associations are shown in bold.
We then assessed the variance explained by each of the 4 psychosocial stressors, i.e. discrimination, childhood trauma, stressful events and low social capital (Environmental models). Each psychosocial stressor was associated with the three subclinical psychosis dimensions, except the low level of social capital that was not associated with the positive dimension (Fig. 1 and Table 3). Of note, when associated with subclinical dimensions, the variance explained by Environmental models was always higher than the one explained by Genetic models (except, concerning the positive dimension, for the low level of social capital).
Combination of genetic and environmental factors
In the Independent models, for the 3 dimensions, the explained variances were better than in the Genetic models, which was confirmed by the LRT (p-values < 0001 for almost all models, Fig. 1 and Table 3, except for the low level of social capital in the positive dimension).
However, concerning the negative and depressive dimensions, in comparison to Environmental models, the Independent models did not fit better. In other words, adding the PRS-SZ to the Environmental factors did not improve the explained variances of these models. Concerning the positive dimension, the Independent models fitted better than both Genetic and Environment models (LRT: p-values between 0.013 and 0.041, Table 3).
In the Interaction models, no significant GxE interaction was observed: adding a GxE interaction term in the Independent models was associated with modest increases of the explained variance, and no interaction term was significantly associated with one of the 3 subclinical psychosis dimension scores. The LRT confirmed that the Interaction models did not fit better than Independent models (Table 3). The analyses presented in Table 3 were repeated in a sample without imputation of the CAPE, without significant change (see online Supplementary Table 3).
Discussion
In this population-based subjects without psychotic disorders transnational study on the relationships between subclinical psychosis and genetic and environmental (psychosocial stressors) risk factors, the PRS-SZ was associated with the positive dimension but not with the negative and the depressive dimensions. By contrast, the psychosocial stressors were positively associated with the 3 dimensions, except for the low level of social capital, which was not associated with the positive dimension. Moreover, considering the positive dimension, PRS-SZ and psychosocial stressors were independently associated, without GxE interaction, consistently with independent effects of genetic and environmental risk factors.
A genetic psychotic continuum?
The association between the PRS-SZ and the positive dimension is consistent with the hypothesis of an aetiological psychotic continuum, with subclinical psychosis and psychotic disorders sharing aetiological – genetic and environmental – factors (Linscott and van Os, Reference Linscott and van Os2013). This hypothesis could not be verified concerning the other dimensions. A precedent EU-GEI study analysing in controls the relationships between subclinical psychosis and another factor associated with the risk of psychotic disorders, i.e. advanced paternal age, found consistent results: significant association with the positive dimension, but not to negative and depressive dimensions (Schürhoff et al., Reference Schürhoff, Pignon, Lajnef, Denis, Rutten, Morgan, Murray, Leboyer, van Os and Szöke2020). The aetiological psychotic continuum could concern particularly the positive dimension. Indeed, in comparison to the negative and depressive dimensions, the positive symptoms are the most specific of psychotic disorders (Hanssen et al., Reference Hanssen, Peeters, Krabbendam, Radstake, Verdoux and van Os2003). Furthermore, in a study analysing the associations between the PRS-SZ and clinical dimensions among antipsychotic-naïve patients with first episode of psychotic disorders (FEP), Santoro et al. (Reference Santoro, Ota, de Jong, Noto, Spindola, Talarico, Gouvea, Lee, Moretti, Curtis, Patel, Newhouse, Carvalho, Gadelha, Cordeiro, Bressan, Belangero and Breen2018) found an association with the positive dimension of the positive and negative syndrome scale (PANSS). Moreover, Markota et al. (Reference Markota, Coombes, Larrabee, McElroy, Bond, Veldic, Colby, Chauhan, Cuellar-Barboza, Fuentes, Kung, Prieto, Rummans, Bobo, Frye and Biernacka2018) found higher level PRS-SZ in manic-psychosis among patient with bipolar disorder. In future studies, it would be interesting to analyse the relationships between the negative and the depressive dimensions with other PRS (e.g. for depression, or bipolar disorder).
Several studies have found an association between the PRS-SZ and subclinical psychosis, but not all. Indeed, some of these studies did not find any significant associations (Derks et al., Reference Derks, Vorstman, Ripke, Kahn, Consortium and Ophoff2012; Nenadić et al., Reference Nenadić, Meller, Schmitt, Stein, Brosch, Mosebach, Ettinger, Grant, Meinert, Opel, Lemke, Fingas, Förster, Hahn, Jansen, Andlauer, Forstner, Heilmann-Heimbach, Hall, Awasthi, Ripke, Witt, Rietschel, Müller-Myhsok, Nöthen, Dannlowski, Krug, Streit and Kircher2020). Methodological differences could be involved, including study population [e.g. some of them were conducted in the paediatric population (Zammit et al., Reference Zammit, Hamshere, Dwyer, Georgiva, Timpson, Moskvina, Richards, Evans, Lewis, Jones, Owen and O'Donovan2014; Pries et al., Reference Pries, Ferro, van Os, Delespaul, Kenis, Lin, Luykx, Richards, Akdede, Binbay, Altınyazar, Yalınçetin, Gümüş-Akay, Cihan, Soygür, Ulaş, Cankurtaran, Kaymak, Mihaljevic, Petrovic, Mirjanic, Bernardo, Mezquida, Amoretti, Bobes, Saiz, García-Portilla, Sanjuan, Aguilar, Santos, Jiménez-López, Arrojo, Carracedo, López, González-Peñas, Parellada, Maric, Atbaşoğlu, Ucok, Alptekin, Saka, Arango, O'Donovan, Tosato, Rutten and Gülöksüz2020a)], or the tools used to measure subclinical psychosis [e.g. schizotypy scales do not take account of hallucinations (Seiler et al., Reference Seiler, Nguyen, Yung and O'Donoghue2020)]. In a recent study from EU-GEI WP6 (‘Vulnerability and Severity’) sample, van Os et al. (Reference van Os, Pries, Delespaul, Kenis, Luykx, Lin, Richards, Akdede, Binbay, Altınyazar, Yalınçetin, Gümüş-Akay, Cihan, Soygür, Ulaş, Cankurtaran, Kaymak, Mihaljevic, Petrovic, Mirjanic, Bernardo, Cabrera, Bobes, Saiz, García-Portilla, Sanjuan, Aguilar, Santos, Jiménez-López, Arrojo, Carracedo, López, González-Peñas, Parellada, Maric, Atbaşoğlu, Ucok, Alptekin, Saka, Arango, O'Donovan, Rutten and Gülöksüz2020) did not find any associations between PRS-SZ and the 3 dimensions of the CAPE in the controls (without psychotic disorders), although among siblings, a significant association with the negative dimension was found. Among the different studies on association between the PRS-SZ and subclinical psychosis, UK Biobank represents the most closely related to EU-GEI (sample from the general population from United Kingdom), and two of the three studies conducted in UK Biobank found significant associations (Legge et al., Reference Legge, Jones, Kendall, Pardiñas, Menzies, Bracher-Smith, Escott-Price, Rees, Davis, Hotopf, Savage, Posthuma, Holmans, Kirov, Owen, O'Donovan, Zammit and Walters2019; García-González et al., Reference García-González, Ramírez, Howard, Brennan, Munroe and Keers2020), contrary to the third, that did not find any significant difference (Alloza et al., Reference Alloza, Blesa-Cábez, Bastin, Madole, Buchanan, Janssen, Gibson, Deary, Tucker-Drob, Whalley, Arango, McIntosh, Cox and Lawrie2020). Of note, in these 3 UK Biobank studies, the samples were different, especially according to the available data of each subject (e.g. MRI data).
Association between environmental and genetic factors
Several studies found G-E associations. In a transnational study (Australia, Netherlands and United Kingdom), the PRS-SZ was associated with the population density of the residence (Colodro-Conde et al., Reference Colodro-Conde, Couvy-Duchesne, Whitfield, Streit, Gordon, Kemper, Yengo, Zheng, Trzaskowski, de Zeeuw, Nivard, Das, Neale, MacGregor, Olsen, Whiteman, Boomsma, Yang, Rietschel, McGrath, Medland and Martin2018), i.e. with urbanicity (Vassos et al., Reference Vassos, Pedersen, Murray, Collier and Lewis2012). These findings were replicated recently in the United Kingdom (Maxwell et al., Reference Maxwell, Coleman, Breen and Vassos2021). Of note, this last study considered also other PRS (for depression, bipolar disorder, etc.) and found analogous results. Other studies found similar associations with the cannabis use (Gage et al., Reference Gage, Jones, Burgess, Bowden, Smith, Zammit and Munafò2017; Pasman et al., Reference Pasman, Verweij, Gerring, Stringer, Sanchez-Roige, Treur, Abdellaoui, Nivard, Baselmans, Ong, Ip, van der Zee, Bartels, Day, Fontanillas, Elson, de Wit, Davis, MacKillop, Derringer, Branje, Hartman, Heath, van Lier, Madden, Mägi, Meeus, Montgomery, Oldehinkel, Pausova, Ramos-Quiroga, Paus, Ribases, Kaprio, Boks, Bell, Spector, Gelernter, Boomsma, Martin, MacGregor, Perry, Palmer, Posthuma, Munafò, Gillespie, Derks and Vink2018). These studies suggest that the association between these environmental factors and the risk of psychotic disorders could partially be explained by the same genetic factors (Pingault et al., Reference Pingault, O'Reilly, Schoeler, Ploubidis, Rijsdijk and Dudbridge2018). This hypothesis particularly concerns childhood trauma, that has often be supposed to be associated with vulnerabilities to psychiatric disorders (Etain et al., Reference Etain, Henry, Bellivier, Mathieu and Leboyer2008; Varese et al., Reference Varese, Smeets, Drukker, Lieverse, Lataster, Viechtbauer, Read, van Os and Bentall2012; Baudin et al., Reference Baudin, Szöke, Richard, Pelissolo, Leboyer and Schürhoff2017). Sharing the same genetic risk factors could explain the association between childhood trauma and psychiatric disorders. However, in our study, we did not find any G-E association neither with childhood trauma nor with the other psychosocial stressors.
Gene x environment (psychosocial stressors) interactions
Our study did not show any statistically significant interaction between the psychosocial stressors and the PRS-SZ, but independent effects concerning the positive dimension. Trotta et al. (Reference Trotta, Iyegbe, Forti, Sham, Campbell, Cherny, Mondelli, Aitchison, Murray, Vassos and Fisher2016) found similar results: the PRS-SZ and childhood trauma history predicted both psychosis status, without interaction between these factors. To our knowledge, two other studies have looked for such interactions, that found a significant GxE interaction between the PRS-SZ and childhood trauma (Pries et al., Reference Pries, Klingenberg, Menne-Lothmann, Decoster, van Winkel, Collip, Delespaul, Hert, Derom, Thiery, Jacobs, Wichers, Cinar, Lin, Luykx, Rutten, van Os and Gülöksüz2020b; Schick et al., Reference Schick, van Winkel, Lin, Luykx, de Zwarte, van Eijk, Myin-Germeys and Reininghaus2022). Another study using the PRS-SZ and conducted in adults looked for interaction with other environmental factors, i.e. smoking status, without finding any GxE interaction (García-González et al., Reference García-González, Ramírez, Howard, Brennan, Munroe and Keers2020).
One hypothesis to explain the negative results of this GxE interaction study is that the PRS-SZ is not the appropriate tool for the study of GxE interaction in psychosis (Assary et al., Reference Assary, Vincent, Keers and Pluess2018). Indeed, this statistical tool summarises essentially monogenic factors with small effects sizes; and GxE interaction could only involve monogenic factors (Caspi et al., Reference Caspi, Moffitt, Cannon, McClay, Murray, Harrington, Taylor, Arseneault, Williams, Braithwaite, Poulton and Craig2005; Stefanis et al., Reference Stefanis, Henquet, Avramopoulos, Smyrnis, Evdokimidis, Myin-Germeys, Stefanis and van Os2007; Alemany et al., Reference Alemany, Arias, Aguilera, Villa, Moya, Ibáñez, Vossen, Gastó, Ortet and Fañanás2011). However, other studies used PRS and found GxE interaction, for instance between childhood trauma and the PRS for depression in the risk of major depressive disorder (Peyrot et al., Reference Peyrot, Milaneschi, Abdellaoui, Sullivan, Hottenga, Boomsma and Penninx2014), or between this PRS and stressful life events in the level of depressive symptoms (Domingue et al., Reference Domingue, Liu, Okbay and Belsky2017), as well as studies on non-psychiatric diseases, e.g. for breast cancer (Meisner et al., Reference Meisner, Kundu and Chatterjee2019; Shi et al., Reference Shi, O'Brien and Weinberg2020). The problem could concern specifically PRS-SZ, with (i) an insufficient sample of subjects included in the genome-wide association studies (GWAS) used to calculate it, which is a major issue concerning PRSs (Plomin and von Stumm, Reference Plomin and von Stumm2018), and (ii) the fact that the PRS-SZ performed better among European descendants (which has prevented the inclusion of subjects from ethnic minorities) (Vassos et al., Reference Vassos, Di Forti, Coleman, Iyegbe, Prata, Euesden, O'Reilly, Curtis, Kolliakou, Patel, Newhouse, Traylor, Ajnakina, Mondelli, Marques, Gardner-Sood, Aitchison, Powell, Atakan, Greenwood, Smith, Ismail, Pariante, Gaughran, Dazzan, Markus, David, Lewis, Murray and Breen2017). Moreover, the PRS does not take copy number variant (CNVs) or epigenetic factors in account, and they are associated with the risk of schizophrenia (and with childhood trauma concerning epigenetic factors) (St Clair, Reference St Clair2009; Shorter and Miller, Reference Shorter and Miller2015; Parade et al., Reference Parade, Huffhines, Daniels, Stroud, Nugent and Tyrka2021). Another hypothesis states that the genes that increase the sensibility to environmental stressors could be different from the genes that increase the risk of schizophrenia (displayed in the GWAS). Furthermore, GxE interactions could also concern other environmental factors (urbanicity, advanced paternal age, migration, etc.). Finally, the study GxE interactions using exposome scores (Pries et al., Reference Pries, Ferro, van Os, Delespaul, Kenis, Lin, Luykx, Richards, Akdede, Binbay, Altınyazar, Yalınçetin, Gümüş-Akay, Cihan, Soygür, Ulaş, Cankurtaran, Kaymak, Mihaljevic, Petrovic, Mirjanic, Bernardo, Mezquida, Amoretti, Bobes, Saiz, García-Portilla, Sanjuan, Aguilar, Santos, Jiménez-López, Arrojo, Carracedo, López, González-Peñas, Parellada, Maric, Atbaşoğlu, Ucok, Alptekin, Saka, Arango, O'Donovan, Tosato, Rutten and Gülöksüz2020a), that takes account of several environmental exposures (including psychosocial stressors), could be instructive.
Limitations
Some limitations should be acknowledged. First, due to the cross-sectional nature of EU-GEI study, the assessment of both subclinical psychosis and psychosocial stressors was retrospective, thus susceptible to be biased (e.g. recall bias) and influenced by clinical variables as depressive or positive symptoms (MacDonald et al., Reference MacDonald, Thomas, MacDonald and Sciolla2015). These potential biases, especially concerning psychosocial stressors assessment (particularly the low level of discrimination experience), have been discussed previously (Pignon et al., Reference Pignon, Lajnef, Kirkbride, Peyre, Ferchiou, Richard, Baudin, Tosato, Jongsma, de Haan, Tarricone, Bernardo, Velthorst, Braca, Arango, Arrojo, Bobes, Del-Ben, Di Forti, Gayer-Anderson, Jones, La Cascia, Lasalvia, Menezes, Quattrone, Sanjuán, Selten, Tortelli, Llorca, van Os, Rutten, Murray, Morgan, Leboyer, Szöke and Schürhoff2021). Moreover, regarding the sample size, that could be considered as insufficient to enhance an GxE interaction, Pries et al. (Reference Pries, Ferro, van Os, Delespaul, Kenis, Lin, Luykx, Richards, Akdede, Binbay, Altınyazar, Yalınçetin, Gümüş-Akay, Cihan, Soygür, Ulaş, Cankurtaran, Kaymak, Mihaljevic, Petrovic, Mirjanic, Bernardo, Mezquida, Amoretti, Bobes, Saiz, García-Portilla, Sanjuan, Aguilar, Santos, Jiménez-López, Arrojo, Carracedo, López, González-Peñas, Parellada, Maric, Atbaşoğlu, Ucok, Alptekin, Saka, Arango, O'Donovan, Tosato, Rutten and Gülöksüz2020a) found an interaction between childhood adversity and PRS-SZ concerning subclinical psychosis (with an ecological momentary assessment) with a lower sample (n = 593). The absence of subjects from ethnic minorities, is a major limitation (Tortelli et al., Reference Tortelli, Nakamura, Suprani, Schürhoff, Van der Waerden, Szöke, Tarricone and Pignon2018). Indeed, these minorities are exposed to higher levels of psychosocial stress (Hatch et al., Reference Hatch, Gazard, Williams, Frissa, Goodwin and Hotopf2016). Contrary to the CAPE (Pignon et al., Reference Pignon, Peyre, Ferchiou, van Os, Rutten, Murray, Morgan, Leboyer, Schürhoff and Szöke2019b), concerning the assessment of these psychosocial stressors, the cross-national invariance of the different tools that were used (CTQ, Williams' major experiences of discrimination scale, LTE, SEAT) has not been studies. Moreover, as the sampling was not fully at random, we cannot assume that our sample was representative of the general population.
Conclusion
This general population-based study revealed an association between PRS-SZ and the subclinical positive dimension of psychosis, as well as independent effects of the PRS-SZ and of the psychosocial stressors (childhood trauma, stressful life events, self-reported discrimination experiences) on the positive dimension, contrary to the negative and depressive dimensions. Moreover, concerning the 3 dimensions, this study did not evidence any GxE interaction, or any G-E association.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796022000464.
Data
Data is confidential and not available.
Acknowledgements
None.
Conflict of interest and Financial support
Dr Bernardo has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Angelini, Casen Recordati, Janssen-Cilag, Menarini, Rovi and Takeda. Dr Arango has received support by the Spanish Ministry of Science and Innovation. Instituto de Salud Carlos III (SAM16PE07CP1, PI16/02012, PI19/024), co-financed by ERDF Funds from the European Commission, ‘A way of making Europe’, CIBERSAM. Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), European Union Structural Funds. European Union Seventh Framework Program under grant agreements FP7-4-HEALTH-2009-2.2.1-2-241909 (Project EU-GEI) and FP7-HEALTH-2013-2.2.1-2-603196 (Project PSYSCAN); and European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking (grant agreement No 115916, Project PRISM, and grant agreement No 777394, Project AIMS-2-TRIALS), Fundación Familia Alonso and Fundación Alicia Koplowitz. Dr James B. Kirkbride has received consultancy fees from Roche and the Health Services Executive, Ireland. He is supported by the National Institute of Health Research University College London Hospital Biomedical Research Centre.
Ethical standards
Ethical approval was obtained from local research ethics committees in each country. The EU-GEI Project was funded by the European Community's Seventh Framework Program under grant agreement no. HEALTH-F2-2010-241909.








