In many developed countries, involving patients in research and decision making is expected. In the United Kingdom, most health research funders require patient involvement in the research process including priority setting, the development of grant applications and as key members of research project teams. A government funded program, INVOLVE (www.invo.org.uk), supports active public involvement in National Health Service (NHS), public health, and social care research and in March 2015, a review of public involvement in government funded research was published (1). This review included a 10-year vision and strategic plan to further increase involvement in research by people using health and social care, and by members of the public.
The need for patient and public involvement and engagement (PPIE) in Health Technology Assessment (HTA) has been recognized for some time with many good practice examples collated by Health Technology Assessment international's (HTAi) Patient and Citizen Involvement Interest Sub-Group (2). However, PPIE in HTA and research in general, whether as recipients of information, as indirect consultants or as direct participants presents barriers that require drivers for success (Reference Gagnon, Desmartis and Lepage-Savary3–Reference Rashid, Thomas, Shaw and Leng9). Strategies for overcoming barriers have been proposed and include sharing resources, well targeted consultation, rigorous qualitative research techniques, clear objectives for consultation, training, feedback and mentoring for patients, neutrality in selecting patients, practical attendance needs (Reference Gagnon, Desmartis and Lepage-Savary3;Reference Gagnon, Desmartis and Gagnon4), attitudes of researchers and the perceived importance of patient involvement (Reference Snape, Kirkham and Britten7).
Specific to HTA, frameworks and submission templates with guidance have been developed for patient groups which offer strategies to drive successful patient involvement (10–Reference Abelson, Wagner and DeJean12). The most recent framework has proposed the inclusion of horizon scanning topic identification as an early opportunity for PPIE (Reference Abelson, Wagner and DeJean12) with PPIE forming the questions to be investigated (Reference Moreria13).
Despite evidence of progress in PPIE in the field of HTA there have been no published developments in early awareness and alert (EAA) systems or horizon scanning activities; an area integral to the HTA process in many healthcare systems. This study is the first to record experiences, impacts, and methods for PPIE in an EAA system.
The National Institute for Health Research (NIHR) Horizon Scanning Research & Intelligence Centre (HSRIC) was until April 2017 an EAA system in England that supplied information to key policy and decision makers in the NHS and the NIHR about emerging health technologies, up to 3 years before launch on the NHS (http://www.hsric.nihr.ac.uk/). HSRIC produced a variety of outputs on single and multiple emerging health technologies (Table 1). Scientific and clinical experts had been involved in these processes from the outset. Driven by a culture to involve and engage patients in research, and having identified the potential benefits to customers of producing outputs that incorporate views of both health professionals and users on aspects of emerging health technologies such as acceptability and impact, HSRIC worked toward involving patients in these processes.
Table 1. NIHR Horizon Scanning Research and Intelligence Centre Outputs

HSRIC used the definitions for involvement and engagement developed by INVOLVE (14). HSRIC reinforced its commitment to PPIE with the publication of its first PPIE strategy in June 2013. This was updated in 2015 following a review of progress made against the initial objectives, and reflections from those involved. The core aims of PPIE at HSRIC were: (i) to identify areas of work where HSRIC could build and strengthen mutually beneficial relationships with patients and the public; (ii) to add value to HSRIC outputs; (iii) to ensure accessibility and effective dissemination of outputs; (iv) to ensure PPIE was strategic, meaningful, and appropriate.
In this study we provide examples of PPIE undertaken at HSRIC and detail the benefits and challenges of this work.
METHODS
HSRIC used recognized methods for established EAA systems; identification, filtration, prioritization, assessment, and dissemination (15). The Centre incorporated PPIE into each of these stages (Figure 1).
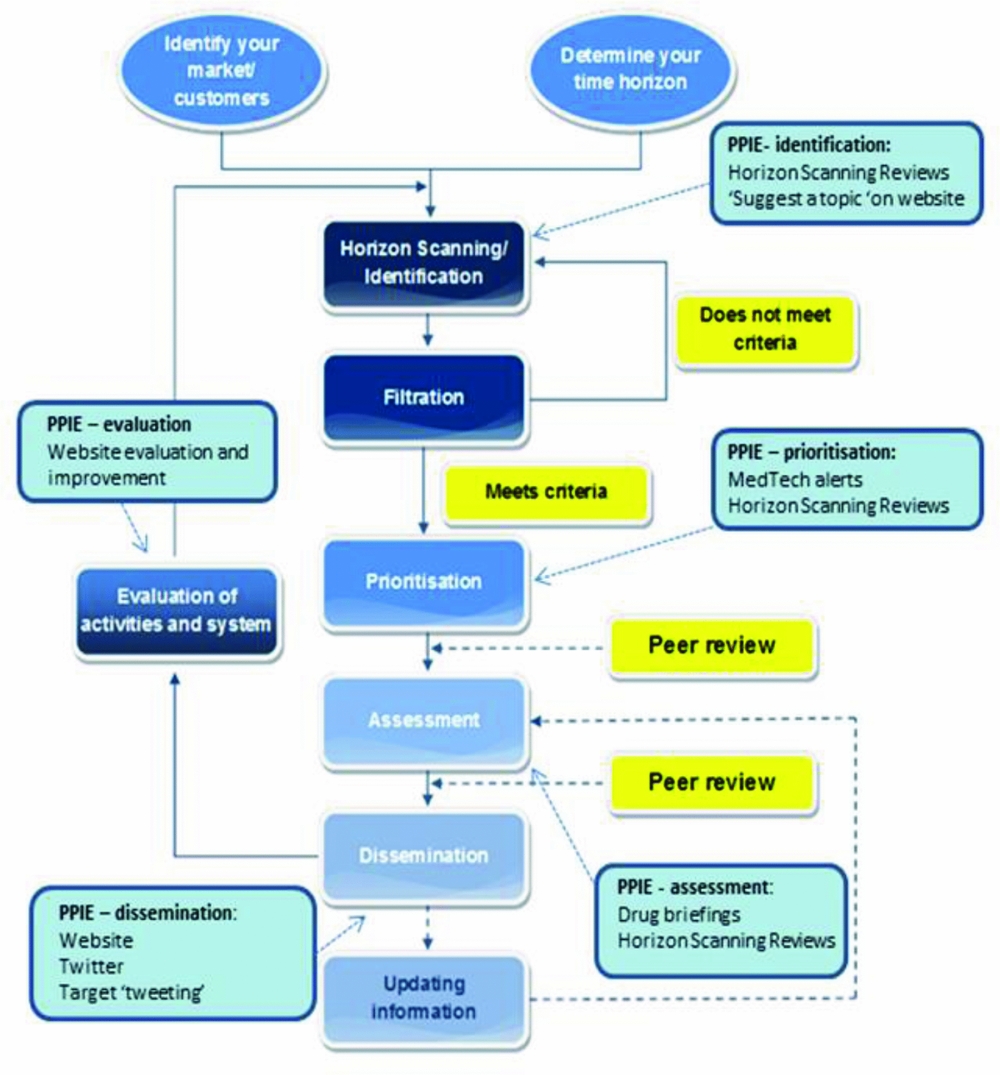
Figure 1. Incorporating patient and public involvement and engagement (PPIE) into early awareness and alert activities, Horizon Scanning Research & Intelligence Centre examples. Modified from: EuroScan International Network. EuroScan International Network, A toolkit for the identification and assessment of new and emerging health technologies, 2014, EuroScan International Network: Birmingham.
Identification
Identification (or horizon scanning) involved finding emerging health technologies that met the remit of HSRIC. This was partly a desktop exercise, searching a range of online sources but also involved liaison with technology developers and clinical experts. At HSRIC, patients and the public were able to contribute to identification in two ways: (i) Suggesting a technology to the Centre through the HSRIC Web site. The Web page was located directly from the main menu and presented a series of text boxes for completion: name, email, telephone, and name of technology were obligatory fields. (ii) Through the process for horizon scanning reviews (Table 1), where patients and carers were asked by email correspondence or during focus groups, if they were aware of any emerging technologies relevant to the review that HSRIC had not already identified.
Filtration and Prioritization
At the filtration stage, technologies found at the identification stage were considered, and by applying pre-set criteria, technologies relevant to HSRIC were selected. Criteria included timeframe to launch in the NHS, level of innovation, and/or potential for impact on patients and health services. Once technologies relevant to the EAA system had been filtered, the remaining technologies could be prioritized according to the system's capacity for assessment of the technologies, customer requirements, and clinician input. At HSRIC, patients were involved when there was uncertainty about the potential for impact and a patient's view was believed to add value to the prioritization of a technology for inclusion in MedTech alerts and horizon scanning reviews. This was particularly important when quality of life or acceptability were thought to be a key factor in the technologies potential for diffusion.
Assessment
At HSRIC, assessment involved presenting a view on the potential impact of emerging health technologies. Patients and carers were invited to comment on drug briefings, MedTech alerts, and on the potential impact, particularly regarding quality of life, of the technologies presented in horizon scanning reviews.
Dissemination
Non-confidential versions of all HSRIC outputs (Table 1) were disseminated on the Centre's Web site. In addition, since 2011, with the increasing importance of social media, HSRIC used Twitter as a means of engagement with the target tweeting of outputs beginning in May 2013 to potentially interested groups including patient organizations and charities.
RESULTS
Examples of PPIE activity and results at each stage of the EAA process outlined in the methods section are presented.
Identification
1. Suggest a Topic – through HSRIC's Web Site
From August 2014 until the end of July 2015, four suggestions were received through the “Suggest a Topic” facility. Two were from commercial developers, one from a communications agency, and one from a clinician. None were from members of the public. Following the launch of a new HSRIC Web site at the end of July 2015 until the end of July 2016, thirteen suggestions were received: six from developers, four from clinicians, one from a member of the public, and two of unknown origin.
2. Horizon Scanning Reviews
For horizon scanning reviews, patients and carers were invited to tell HSRIC about technologies they knew about that were in development. An example is a review carried out to identify new and emerging technologies for inherited retinal disease in 2014, a topic highlighted through the James Lind Alliance Sight Loss and Vision Priority Setting Partnership. Patients and carers contributed with information about three emerging technologies that they were aware of that had not been identified by HSRIC. These were the use of human induced pluripotent stem cells to generate synthetic retinae, replacement lens for patients affected by retinitis pigmentosa, and a nutritional complex aimed at retinal conditions. These topics were investigated further but did not meet the review's inclusion criteria as they were either for indications outside the relevant disease area, or had not progressed past animal trials.
Filtration/Prioritization
Assistance in the Prioritization of a Medical Device
In May 2015, the MedTech team identified a wearable technology designed to reduce acid reflux into the throat and lungs and the associated symptoms of laryngopharyngeal reflux disease. The device, a band worn round the neck like a collar, worked by applying a slight pressure at the cricoid cartilage region. This increases the luminal pressure within the upper esophageal sphincter and, thereby, stops the regurgitation of stomach contents from rising above the upper esophageal sphincter.
Patient input was sought on the potential for impact of this technology and particularly views on the device's acceptability. The technology was described using available information, and patients were asked whether they would use it if it were available. Initial emails requesting input were sent out to two charities, FORT (Fighting Oesophageal Reflux Together) and Living with Reflux, which led to a recommendation to contact Action Against Heartburn (www.actionagainstheartburn.org.uk). Action Against Heartburn posted a request for feedback on the technology on their Web site and forwarded our request to the Chairman of Barrett's Wessex (www.barrettswessex.org.uk), a patient support charity that aims to raise awareness of Barrett's esophagus, who contacted patients of his group who subsequently contacted HSRIC directly.
Comments were received from five individuals. Four of the people who responded expressed their wish to try this device if it became available. Patient interest to try a device that appeared undesirable to HSRIC and clinicians highlighted this technology as high priority for assessment. We, therefore, pursued the technology and completed a MedTech alert that was posted on our Web site in September 2015 (16).
Assessment
Comments on a Drug Briefing
In March 2014, HSRIC were approached by Genetic Alliance UK, an umbrella patient group for genetic diseases who offered to work as a conduit between HSRIC and an appropriate patient group on any genetic topics on which HSRIC were producing briefings. In April 2014, a suitable topic was identified to pilot the input of patient comments; recombinant human alpha-mannosidase for alpha-mannosidosis, a rare enzyme deficiency disorder which results in defective mannosidase activity. This accumulation causes problems with bone, cartilage skin, and tendon development (17).
A briefing draft was produced by HSRIC and the Genetic Alliance UK facilitated comments from the Society for Mucopolysaccharide Diseases (MPS Society). The MPS Society made contact with most of the 14 patients with alpha-mannosidosis in the United Kingdom. As engaged patients who had become experts in their rare disease, they were able to provide some replacement paragraphs which were more up to date than the information identified in HSRIC's Web searches, for example in terms of the presentation of the disease. Most of the comments made by the patient group (Table 2) were incorporated into the briefing, which was posted on HSRIC's Web site in June 2014 (18).
Table 2. Patient Group Contribution to a Drug Briefing

UK MPS, United Kingdom Society for Mucopolysaccharide Disease; NHS, National Health Service.
Input into Horizon Scanning Reviews
Eight horizon scanning reviews involved patients and carers. These reviews identified new and emerging health technologies for urinary and faecal incontinence, inherited retinal diseases, chronic obstructive pulmonary disease, corneal diseases, epilepsy, and hearing loss, and have investigated developments in artificial pancreas device systems and non-invasive glucose monitoring technologies. For each of these reviews, a table of identified emerging technologies with a description of each technology and information on development has been presented to patients, carers, or representatives of these groups. In most cases, this has been sent electronically by email but printed tables have been sent and on a couple of occasions the information has been delivered as part of a focus group.
The way in which patients have been engaged in the process, and the type of patients involved has varied. Generally previously established groups such as national patient support organizations or research charities have initially been contacted. These have either referred us to individual patients, acted as an intermediary between HSRIC and patients, or responded on behalf of the patients. Patients have often been those that may be referred to as expert patients, ready-made groups of patients who respond to invitations to be involved in research.
Continuing with the example horizon scanning review of new and emerging technologies for inherited retinal diseases, forty technologies were identified as meeting the review's inclusion criteria. These technologies were presented to two focus groups consisting of patients and carers in a format suitable for this group (i.e., large print and using appropriate software) and considered for their potential utility and impact.
The focus groups were arranged and facilitated by the U.K. charity Fight for Sight. Adaptations were made by Fight for Sight to the materials prepared for consideration by the groups so that they were suitable for those people that were partially sighted. The patients who participated were expert patients with a good knowledge of their disease area. Fight for Sight fed back to HSRIC that some people had difficulties with understanding the technical information sent by HSRIC, yet they were able to comment on many of the technologies. Patients also included more general comments on services, payment, and adoption issues of the new technologies; these provided additional insights to the technology area and were incorporated into the final report.
Dissemination
Involving Patients in Reviewing the HSRIC Website
In January 2014, we reviewed two discrete HSRIC Web site functions to consider user-friendliness of the “Suggest a topic” page and the “Search HSRIC” facility for HSRIC reports and outputs. HSRIC engaged with a local, pre-existing patient group, the Birmingham Rheumatology Research Patient partnership (R2P2), who had previous experience of Web site related activities. In April 2014, facilitated by HSRIC, the Web site review focus group (three members of R2P2) met. In order for the focus group to be a success, attention was paid to ensure practical details were in place: a map of the venue, disabled parking reserved, refreshments provided, provision of a laptop per person, a program of timings for the participants, reimbursement of travel expenses, and a voucher offered for time. Care was taken to introduce the role of the HSRIC in “jargon free” language and the session was led ensuring all participants contributed fairly. Flip chart notes were taken throughout the session so the participants could see what was being recorded. After the session, a report was produced and sent to the patients for validation.
For the “Suggest a topic” page, comments from the group included: (i) The introductory first paragraph needs to “grab you” more with bullet points; (ii) There needs to be an indication of who gets the form once it is submitted; (ii) Could the font be more “friendly”?
For the “Search HSRIC” facility page, comments from the group included: (i) a help facility to aid with searching function; (ii) improved clarity on the presentation of the search results to indicate which part of the results were on show; (iii) linking more lay terms for diseases to medical names, for example, “stomach cancer” linked to “gastrointestinal cancer.” A general request was that they would like to see a flow diagram of HSRIC's processes somewhere on the Web site.
In November 2015, we conducted a second focus group with eight members of the public who volunteered at the Queen Elizabeth Hospital in Birmingham. The purpose of the session was to ask for their thoughts and feedback on aspects of the HSRIC's new Web site (launched summer 2015), including the improved search and “suggest a topic” facilities and the new “for the public” pages. The session was based on the basic methods of the 2014 group and built on practical lessons learnt from running that session. It was again interactive with easy access to the Web site for all, with short exercises followed by facilitator led feedback. The session generated lots of useful comments from the participants that HSRIC implemented in 2016, including: (i) simplifying text on the “for the public” pages, (ii) changing fonts and colors on the “search facility” for improved ease of use, (iii) explanation on the use of filters in the “search facility.”
The issues raised from both focus groups were incorporated in to the new HSRIC Web site unless they were outside the scope of the Centre. For example, there was a request to link to medical advice but this was believed to add a complexity to the Web site that would be difficult to quality assure.
Engaging Patients and the Public by Disseminating Outputs Using Twitter
From June/July 2011 all drug briefings, MedTech alerts and horizon scanning reviews were disseminated using Twitter; an easy and convenient way to reach patients and the public. Since May 2013 reports have also been “target tweeted” at relevant patient groups when appropriate and a suitable patient group can be identified. By the end of July 2016, a total of 227 target tweets had been sent potentially increasing access to the interested public who would otherwise not directly receive outputs.
DISCUSSION
HSRIC has involved or engaged patients and the public at all stages of the EAA system and for all types of output. Patients have been very positive about their involvement and potential to influence at an early stage in a health technologies development. We speculate that this is because as experts in their own illness they are been given the opportunity to input and potentially have an impact, and that their contribution may help in further development and prioritization of important technologies.
We believe that, where PPIE has been implemented, it has allowed the Centre to produce outputs that enable a better understanding of the needs of possible users in relation to the presented emerging health technologies; ad hoc feedback from decision makers has suggested this is the case. It has informed decision makers and those evaluating technologies about relevant emerging technologies that are acceptable to patients and are likely to have the greatest impact on their quality of life; it has also highlighted in the case of horizon scanning reviews where further research may be required.
PPIE at an early stage in a technology's development could potentially increase the chances of successful adoption and implementation of health technologies, and even improve innovations. Disseminating, and increasing the accessibility of, information on emerging health technologies to patients and carers will also increase their awareness of emerging technologies and enable improved engagement in decision making, be it about their own healthcare situation or in contributing to the wider health system. We found that incorporating PPIE into EAA activities was more successful at some stages than others.
a. Identification
Although providing an opportunity for patients and the public to input into an EAA system by enabling them to alert the system to emerging health technologies is relatively easy and requires few resources, we found that this to be the least valuable aspect of PPIE in our system. It is clear from the results that the “suggest a topic” facility was not a productive identification source of technologies for the Centre, from members of the public or otherwise. The underuse of this facility could be due to several factors, including lack of awareness of the facility within the Web site and of EAA activities in general.
Additional factors may be that they are not aware of technologies that fit our remit, or are reticent to propose technologies as they are unsure of their relevance and interest to HSRIC. The direct approach in horizon scanning reviews also yielded very few relevant suggestions possibly due to lack of understanding of the process. Patients in particular can be a wealth of knowledge on their disease and potential treatments but may not be aware of technologies on the far horizon. Another possibility is that many interested patients have been educated in the need for good evidence and as emerging technologies do not have this at any early stage the patients may be reluctant to share uncertain information.
b. Filtration/Prioritization
We found involving patients with the prioritization of medical technologies to be advantageous. In our experience patients add their own valuable perspectives to the viewpoint of the technology's potential for impact. Areas where they will have particular insight include views on quality of life, acceptability, and ease of use. Areas we found challenging with PPIE at this stage included: (i) the dearth of information usually available about health technologies at an early stage in their development, this means that it can be difficult to present patients with the level and type of information they will find useful; (ii) deciding on what technologies to ask patients to provide comment on. HSRIC typically considered approximately 180 medical technologies per year at the prioritization stage. Involving patients in prioritization for all of these may be difficult as there is often little information on the emerging technology on which to base a decision. However, it could be argued that the decision on which technologies we should ask for comment on should be determined by the patients themselves.
c. Assessment
Working with patient groups to comment on drug briefing reports and horizon scanning reviews has added new information, identified unnecessary and inaccurate information, explained the context of current treatment options, explained the impact of symptoms and treatments on the patient and associated costs, and added valuable information on acceptability of emerging health technologies. Future horizon scanning assessment work would benefit from patient comment where: (i) little available published information on the epidemiology of the disease is available. This may be most helpful for rare conditions (ii) where the patient group is likely to hold disease registers; (iii) when there are many existing therapies and it is not clear what the benefit of a new therapy is, and patient preference and the outcomes patients value are likely to play an important role; (iv) where there are many emerging technologies and input can help to single out the most promising technologies from the view of a patient.
d. Dissemination
Involving the public in a review of our Web site, through a focus group approach, was generally successful with many insightful comments made. The public offer an objectivity and freshness to the subject at hand that cannot be achieved by internal staff or specialists. The focus groups take a significant amount of time to organize and require a careful and considered approach on the day. The facilitator has to ensure that individuals do not get side tracked into irrelevant discussions and understand enough of what an EAA system does to input usefully in to a review of its Web site. Personalities need to be handled with diplomacy and attention paid to the quieter members of the group to ensure a balance of opinion.
There are several challenges to incorporating PPIE into an EAA system. A key barrier could be attributed to resources required and the challenges of measuring the impact of such involvement in both the short- and long-term (Reference Mockford, Staniszewska, Griffiths and Herron-Marx19). This aside, there are more tangible challenges, including logistical issues regarding the tight turnaround required for producing outputs, the lack of familiarity with the often technical nature of the emerging health technologies and EAA systems themselves, the dearth of information available on new health technologies, the terminology involved in health services research, the skills required by researchers to engage with patients being different to existing skill sets, and ensuring a balance between an output that is suitable for health professionals and policy makers and one that is accessible to the general public.
We are not alone in these challenges and a survey conducted in 2010 of members of the International Network of Agencies for Health Technology Assessment (INAHTA) of the involvement of “consumers” in their programs, found that there was a trend of increased involvement compared with an earlier survey of 2005, but that the level of involvement was relatively limited (Reference Hailey, Werkö and Bakri5). Others have highlighted the need for significant development of the PPIE evidence base, particularly around guidance for the reporting of user activity and impact (Reference Mockford, Staniszewska, Griffiths and Herron-Marx19). The feasibility of patient engagement in many healthcare research settings requires extra time and funding needed for engagement and the concern over tokenism (Reference Domecq, Prutsky and Elraiyah6).
Implications for Policy and Practice
HSRIC has demonstrated that involvement and engagement of patients and the public in all aspects of EAA activities is possible (Figure 1) and can have many benefits. EAA systems should consider embedding PPIE into their activities and allocating resources. This may require a dedicated post with experience in the field to champion PPIE in the organization, and establishing a patient and public user group to advise and challenge the EAA on all aspects of PPIE, including leading on what technologies we should get involvement on. In addition, training and new materials for staff and members of the public involved will be required to ensure understanding of the process.
CONCLUSION
EAA systems should consider involving and engaging with patients and the public in identification, filtration, prioritization, and assessment of emerging technologies where resources allow. Where resources are limited, the focus could be on using PPIE when a technology is for a rare disease and where information and expertise is limited. Further research is required to examine the value and impact of involving and engaging patients and the public in EAA activities and in the early development of health technologies. Gaining views of decision makers and developers where PPIE has been carried out would provide valuable insight.
CONFLICTS OF INTEREST
The authors report grants from National Institute for Health Research, during the conduct of the study.







