Introduction
One of the key aims of studies of host–parasite interactions is understanding host characteristics and environmental factors that influence parasite transmission dynamics. The effects of host sex, age and season of sampling on parasite abundance, prevalence and species richness have been studied repeatedly, often with varying outcomes depending on the host or parasite taxa studied or the spatial or temporal context (Isomursu et al., Reference Isomursu, Rätti, Helle and Hollmén2006; Saeed et al., Reference Saeed, Maddox-Hyttel, Monrad and Kapel2006; Martínez-Guijosa et al., Reference Martínez-Guijosa, Martínez-Carrasco, López-Olvera, Fernández-Aguilar, Colom-Cadena, Cabezón and Serrano2015; Amundson et al., Reference Amundson, Traub, Smith-Herron and Flint2016; Spickett et al., Reference Spickett, Junker, Krasnov, Haukisalmi and Matthee2017).
In recent years, parasite ecologists focused their efforts on the effects of these factors on parasite community structure. Studies aiming to elucidate if parasites live in structured communities or in unstructured assemblages were conducted mainly with a view to parasite communities in fish hosts (Guégan and Hugueny, Reference Guégan and Hugueny1994; Rohde et al., Reference Rohde, Worthen, Heap, Hugueny and Guégan1998; Carney and Dick, Reference Carney and Dick2000; Norton et al., Reference Norton, Lewis and Rollinson2004), amphibians (Zelmer et al., Reference Zelmer, Paredes-Calderón, León-Règagnon and García-Prieto2004; Martins et al., Reference Martins, Poulin and Gonçalves-Souza2021), birds (Calvete et al., Reference Calvete, Estrada, Lucientes, Estrada and Telletxea2003; Amundson et al., Reference Amundson, Traub, Smith-Herron and Flint2016) and small mammals (Warburton et al., Reference Warburton, van der Mescht, Khokhlova, Krasnov and Vonhof2018; Krasnov et al., Reference Krasnov, Shenbrot, Warburton, Van Der Mescht, Surkova, Medvedev, Pechnikova, Ermolova, Kotti and Khokhlova2019; Rynkiewicz et al., Reference Rynkiewicz, Fenton and Pedersen2019; Cardoso et al., Reference Cardoso, Andreazzi, Maldonado Junior and Gentile2021).
However, relatively few studies dealt with parasite communities of large mammalian hosts. This is because it is often difficult to obtain multiple samples of large mammals, since population numbers have shrunk as a consequence of habitat loss due to human activities, and populations are limited to relatively small conservation areas. In addition, the sheer effort of processing large hosts for parasite sampling and identification often proves prohibiting. Nevertheless, 1 such study on a large marine mammal species was conducted by Bellay et al. (Reference Bellay, Oda, Almeida-Neto, de Oliveira, Takemoto and Balbuena2020). These authors investigated the influence of host age and sex on infracommunities in a pilot whale–helminth network and concluded that host age was one of the main drivers of the occurrence and species richness of intestinal helminths. Moreover, they found that the increase in parasite richness within a host resulted mainly from non-random infection with helminths.
Recently, we studied the impact of host sex and age on the structure of individual-based host–parasite networks in nyalas, Tragelaphus angasii Angas, from 3 game reserves in South Africa (Junker et al., Reference Junker, Boomker, Horak and Krasnov2022). We found that the effects of both host sex and age can be mediated by local environmental conditions and may manifest differently in the same host but sampled in different localities. Similar work on wild ruminants was done by Fellis et al. (Reference Fellis, Negovetich, Esch, Horak and Boomker2003) and Negovetich et al. (Reference Negovetich, Fellis, Esch, Horak and Boomker2006) in southern Africa. Fellis et al. (Reference Fellis, Negovetich, Esch, Horak and Boomker2003) found non-randomness in parasite communities of greater kudu, Tragelaphus strepsiceros (Pallas), in the Kruger National Park (KNP) and, comparing these to parasite communities of kudus in Etosha National Park, Namibia, concluded that biogeography and host demographics played an important role in the organization of helminth communities. Negovetich et al. (Reference Negovetich, Fellis, Esch, Horak and Boomker2006) demonstrated the influence of social and feeding behaviour of different age-sex classes of impalas, Aepyceros melampus (Lichtenstein), on parasite communities. These findings may have important consequences for planning and designing parasitological investigations. However, it is still unclear whether environmental mediation of the effects of host-associated factors on parasite community structure found for parasite infracommunities in nyalas, kudus and impalas also holds for other host species.
Comprehensive data sets on the gastrointestinal parasites of common warthogs, Phacochoerus africanus (Gmelin) (Suidae), compiled by Horak et al. (Reference Horak, Boomker and Potgieter1988) and Boomker et al. (Reference Boomker, Horak, Booyse and Meyer1991), allowed us to address this gap. Monogastric warthogs differ both in their sociality and dietary preferences (see below) from the above ruminant species, which are more gregarious and intermediate mixed feeders or browsers (Skinner and Chimimba, Reference Skinner and Chimimba2005). To date, few studies have investigated helminth communities in wild species of suids (Corn et al., Reference Corn, Pence and Warren1985; de-la-Muela et al., Reference de-la-Muela, Hernández-de-Luján and Ferre2001; Foata et al., Reference Foata, Mouillot, Culioli and Marchand2006). Junker et al. (Reference Junker, Spickett, Swanepoel, Krasnov, Boomker and Hoffman2019), having studied the influence of host age and sex on infrapopulations of gastrointestinal helminths of warthogs in the KwaZulu-Natal Province in South Africa, emphasized a need to further explore helminth assemblages and their structure in wild suids in Africa.
Here we studied the effects of host sex, age and season of sampling on the structure of helminth infracommunities harboured by warthogs at 2 localities in South Africa. First and similar to our earlier study on the parasites of nyalas, we searched for non-random structural patterns in the warthog–helminth interaction networks. Among a variety of ecological structures, nestedness (i.e. a pattern where species found in species-poor communities represent a proper subset of species in richer communities) is one of the most common properties of species distributions (Guégan and Hugueny, Reference Guégan and Hugueny1994; Poulin and Valtonen, Reference Poulin and Valtonen2001; Krasnov et al., Reference Krasnov, Stanko, Khokhlova, Shenbrot, Morand, Korallo-Vinarskaya and Vinarski2010; Rynkiewicz et al., Reference Rynkiewicz, Fenton and Pedersen2019). Then, we investigated contributions of male and female young and adult warthogs sampled during dry and wet seasons to this nested pattern. As mentioned above, similar work had been done by Fellis et al. (Reference Fellis, Negovetich, Esch, Horak and Boomker2003). However, Fellis et al. (Reference Fellis, Negovetich, Esch, Horak and Boomker2003) used the matrix temperature as a measure of nestedness (Patterson and Atmar, Reference Patterson and Atmar1986; Atmar and Patterson, Reference Atmar and Patterson1993). This methodology has been subject to severe criticism because this metric is susceptible to type 1 errors and, especially in communities comprising mainly ubiquitous and rare species, the use of this metric might lead to false conclusions (Ulrich et al., Reference Ulrich, Almeida-Neto and Gotelli2009). Here, we applied the most popular nestedness metric, the Nestedness based on Overlap and Decreasing Fill (NODF) index (Almeida-Neto et al., Reference Almeida-Neto, Guimarães, Guimarães, RD and Ulrich2008; Ulrich et al., Reference Ulrich, Almeida-Neto and Gotelli2009). This index is robust to changes in matrix shape or size and can vary from zero in a not nested (=random) network to 100 in a perfectly nested network. One of the advantages of the NODF index is that it quantifies nestedness for not only the whole matrix but also nestedness independently contributed by matrix rows (in our case, nestedness among helminth species) and matrix columns (in our case, nestedness among host individuals).
Secondly, we considered beta diversity of helminth infracommunities harboured by warthogs (i.e. helminth diversity between individual warthogs). Legendre and De Cáceres (Reference Legendre and De Cáceres2013) argued that beta diversity of a set of communities is affected by both individual species and individual species assemblages (=sites) and developed a method that allows to separate these effects. In other words, total beta diversity can be partitioned into (a) the degree of relative importance of individual species for beta diversity across an area (=Species Contributions to Beta Diversity; SCBD) and (b) indicators of the compositional uniqueness of the assemblages as compared to other sites (=Local Contributions to Beta Diversity; LCBD). This partitioning allows a better understanding of the differential roles of species and assemblages in total beta diversity. Here, we focused on LCBD, which in application to parasite communities should be called Host Contributions to Beta Diversity (HCBD), and tested for the associations between warthog sex, age and season of sampling and HCBD to elucidate the reasons behind the compositional uniqueness of helminth infracommunities.
Thirdly, we asked whether warthog sex, age and season of sampling affected dark diversity of their helminth infracommunities. In contrast to realized diversity (i.e. species that are present in a locality), dark diversity (Pärtel et al., Reference Pärtel, Szava-Kovats and Zobel2011) is defined as the assemblage of species from the regional pool that could inhabit this locality due to suitable conditions but that are, in reality, absent. In application to parasite infracommunities, an individual host of a given species is analogous to a locality, whereas parasite species harboured by all hosts of this species in the same geographic region or habitat type are analogous to a regional species pool because they are able to exploit this host species. Estimations of parasite dark diversity allow to identify the gaps between parasite species richness in an individual host and a parasite species pool and comparisons of these gaps (i.e. dark diversity estimates) between individual hosts may allow better predictions and preventions of parasitic disease outbreaks. Estimation of dark diversity requires definition of a species pool, which cannot be arbitrarily established from species inventories because this approach (a) ignores species’ ecological constraints and the interspecific variation in these constraints (Lewis et al., Reference Lewis, Szava-Kovats and Pärtel2016) and (b) inventories, obviously, cannot account for species that are a part of dark diversity.
Thus, as presented above, we sought to uncover the effect of host sex, age and season of sampling on: (1) patterns of nestedness in warthog–helminth networks, (2) the contribution of species assemblages in individual warthogs (HCBD) to total beta diversity, and (3) dark diversity of helminth infracommunities of warthogs.
Finally, we asked whether the effects of host sex, age and sampling season on structural patterns differ between the 2 localities because of local environmental processes. Altogether, answering these questions will allow us to determine internal and external factors that serve as drivers of the community structure of helminths of warthogs.
Materials and methods
Host species
Common warthogs are widespread in sub-Saharan Africa. With exception of the forest zone, their range extends westwards from Senegal to Ethiopia and southwards to South Africa. Within South Africa, warthogs are common in the Limpopo and Mpumalanga Provinces and in the north-eastern parts of North West Province. Their preferred habitats are savanna, grassland, open woodland and bushland, but they also frequent floodplains and wetlands. Warthogs are diurnal and spend the day foraging for grasses, especially those that are fresh and green, herbs, berries and wild fruits. They will also root for underground rhizomes of grasses. Their diet is seasonally variable with more time spent rooting during winter (June to September), whereas grazing is preferred in summer. Males (59.2–103.9 kg) are larger than females (44.6–69.1 kg) (Skinner and Chimimba, Reference Skinner and Chimimba2005). The size of warthog groups is usually small. Adult males are typically solitary, but mingle with other warthogs at waterholes, while younger males of more than 1 year of age join bachelor groups of 2–3 individuals. Matriarchal groups comprise 1 or more females with their juvenile and sometimes yearling offspring, although yearlings eventually move off to form yearling groups that exclude any other age classes (Somers et al., Reference Somers, Rasa and Penzhorn1995). Warthogs are not territorial and groups occupy overlapping home ranges. The mating system is promiscuous, with males moving between the home ranges of several females; females will mate with more than 1 male (Somers et al., Reference Somers, Penzhorn and Rasa1994; Skinner and Chimimba, Reference Skinner and Chimimba2005).
Study areas
Warthogs were collected from 3 sampling sites within the KNP in Mpumalanga Province (see Horak et al., Reference Horak, Boomker and Potgieter1988) and from 1 sampling site in the Hoedspruit Nature Reserve (HNR; now AFB Game Reserve; 24°21’17”S, 31°03’01”) in Limpopo Province, South Africa (see Boomker et al., Reference Boomker, Horak, Booyse and Meyer1991). The sites in the KNP were the areas around the Skukuza (24°59’43”S, 31°35’34”E), Crocodile Bridge (25°21’30”S, 31°35’32”E) and Lower Sabie (25°7’16”S, 31°55’2”E) rest camps. All collection sites are situated in the Savanna Biome in the Lowveld bioregion in a vegetation zone classified as Granite Lowveld (Mucina and Rutherford, Reference Mucina and Rutherford2006). The climate is characterized by hot summers and mild, usually frost-free winters. Winters are dry, with the main rainfall occurring in summer.
Climate data were recorded during the sampling period at Skukuza (January 1980 to January 1981) and in the HNR (August 1988 to July 1989). At Skukuza, a minimum temperature of 3°C was recorded in June 1980 and a maximum of 33°C was reached in January 1981. A total of 748 mm of rain (0–160 mm) was measured in the 13-month period (see Horak et al., Reference Horak, Boomker and Potgieter1988). At HNR, the minimum temperature recorded in July 1989 was 10.4°C, the maximum was 30.7°C in March 1989, with a total of 350 mm of rain (0–108 mm) in the twelve-month period (see Boomker et al., Reference Boomker, Horak, Booyse and Meyer1991).
At the time of sample collection, the HNR comprised approximately 4000 ha, owned by the South African Defense Force. Within this reserve, and covering a restricted area of about 2000 ha, the Air Force Base Hoedspruit was situated. Warthogs were collected in both the open and restricted area of the reserve. A series of security fences between the 2 areas prevented warthogs from either side of the fence from passing through. In contrast, the outer fencing between the HNR and adjacent privately owned game farms, did allow warthogs to pass freely.
Host and parasite collection
A total of 54 warthogs (23 females, 31 males) were collected on a monthly basis from January 1980 to January 1981 in the KNP. Of these, 10 were adult females, 13 young females, 18 adult males and 13 young males. Nineteen warthogs were collected in the dry season (May to August), whereas 35 were collected in the wet season (September to April). With the exception of March 1989, when none could be located, warthogs were collected on a monthly basis from August 1988 to July 1989 at HNR. In total, 41 warthogs (25 females, 16 males) were collected, including 14 adult females, 11 young females, 6 adult males and 10 young males. Of these, 16 were collected during the dry season, 25 in the wet season. Warthogs from 1 to 24 months of age were classified as young animals, and animals >24 months of age as adults.
Warthogs were processed for worm recovery as detailed in Horak et al. (Reference Horak, Boomker and Potgieter1988) and Boomker et al. (Reference Boomker, Horak, Booyse and Meyer1991). Briefly, carcasses were eviscerated and each gastrointestinal tract divided into stomach, small intestine and large intestine. The contents of these sections were removed and aliquots made from the ingesta. In addition, digests and aliquots thereof for the recovery of fourth-stage larvae (Impalaia tuberculata L4 and Trichostrongylus sp. L4) were made of the mucosae of the GIT sections of warthogs in the KNP, but not for warthogs in the HNR. Parasites were collected from the aliquots, identified and counted. Aliquot counts were subsequently converted into full counts. Schistosoma sp. was collected from the mesenteric veins. Parasites were identified using relevant keys and taxonomic works. Species and their authorities are listed in Supplementary Tables 1 & 2.
As it is often difficult to distinguish congeneric females within the Strongylida, we have assigned females in a host individual to the various species based on the ratio of males. If males were absent, females could only be identified to genus level. To date, 2 species of the cestode genus Moniezia Blanchard, 1891 have been described from warthogs, Moniezia mettami Baylis, 1934 and Moniezia phacochoeri (Baylis, 1927) (syn. Paramoniezia phacochoeri Baylis, 1927) (Beveridge, Reference Beveridge2014). Because of time constraints, specimens were only identified to genus level and are treated here as Moniezia sp. Counts concerning Moniezia sp. were based on scoleces. Representatives of the nematode genus Probstmayria Ransom, 1917 recovered from warthogs in South Africa have generally been assigned to Probstmayria vivipara (Horak et al., Reference Horak, Boomker and Potgieter1988; Boomker et al., Reference Boomker, Horak, Booyse and Meyer1991; Junker et al., Reference Junker, Spickett, Swanepoel, Krasnov, Boomker and Hoffman2019). However, during the course of this study, we found the description of Probstmayria suis Troncy, Graber and Thal, 1927 as a new species from P. africanus (as Phacochoerus aethiopicus (Pallas)) and Hylochoerus meinertzhageni Thomas in the Central African Republic (Troncy et al., Reference Troncy, Graber and Thal1972). According to the latter authors, differentiation between the females of the 2 species in the absence of males, which are extremely scarce, is not possible. Since no males could be examined, we record the specimens here as Probstmayria sp. Counts of Probstmayria sp. were in their millions and are therefore not further specified.
Data analysis
An interaction warthog–helminth network for each of the 2 localities was represented by a presence/absence matrix with individual warthogs in columns and helminths in rows. As mentioned above, we tested whether helminth infracommunities of warthogs demonstrated a nested pattern by the NODF index (Almeida-Neto et al., Reference Almeida-Neto, Guimarães, Guimarães, RD and Ulrich2008; Ulrich et al., Reference Ulrich, Almeida-Neto and Gotelli2009). In the context of this study, we focused on nestedness among individual warthogs (i.e. nestedness among matrix columns). We calculated NODF using the program NODF 2.0 (Almeida-Neto and Ulrich, Reference Almeida-Neto and Ulrich2011) and determined its significance using a null model with fixed column (hosts) and equiprobable row constraints (Gotelli, Reference Gotelli2000) with 1000 permutations. This algorithm does not constrain the number of helminth species that can occur in a host individual (see Krasnov et al., Reference Krasnov, Stanko and Morand2006 for justification of this model in application to host–parasite associations). Prior to calculations of NODF, each matrix was sorted according to helminth species richness (i.e. maximally packed matrix). Then, following Wang et al. (Reference Wang, Ding, Chen and Zheng2013) and Chen et al. (Reference Chen, Zhan and Wang2022), we derived the nestedness rank of each individual warthog in the maximally packed matrix from the default output.txt of the NODF program. In addition, for each individual warthog, we calculated Z-transformed nestedness resultant (Nres) and the nestedness contribution (Ncont) (Almeida-Neto and Ulrich, Reference Almeida-Neto and Ulrich2011). The Nres of an individual warthog is the standard effect size of the NODF metric where the observed degree of nestedness is compared to the degree after randomizing the occurrences of this warthog. The Ncont of an individual warthog represents the difference between the NODF metric calculated when this individual was excluded and the NODF metric calculated when it was included. Therefore, it indicates whether the matrix nestedness increases (Ncont < 0) or decreases (Ncont > 0) after this individual is excluded from the calculations.
Then and following Junker et al. (Reference Junker, Boomker, Horak and Krasnov2022), we estimated the importance of each individual warthog in a network using 3 indices. The index of individual host (=warthog) specialization (d’) represents the deviation from a neutral configuration of associations (Blüthgen et al., Reference Blüthgen, Menzel and Blüthgen2006; Blüthgen, Reference Blüthgen2010) by comparison of the frequency distribution of interactions with a null distribution when interactions between warthogs and helminths are proportional to their observed total frequencies. This index may vary from 0 (no specialization) to 1 (complete specialization). Individual host strength (IHS) is a species-strength index of Bascompte et al. (Reference Bascompte, Jordano, Melian and Olesen2003) calculated as the sum of the dependencies of each helminth species on each individual warthog. d’ and IHS were calculated using function ‘specieslevel’ of the R package ‘bipartite’ (Dormann et al., Reference Dormann, Gruber and Fruend2008; Dormann, Reference Dormann2011). Centrality (C) assesses the role of each warthog in sharing helminths with other warthogs within a locality. High centrality of a warthog would indicate its greater connection with other warthogs thus would suggest high level of helminth transmission (Morand et al., Reference Morand, McIntyre and Baylis2014; Pilosof et al., Reference Pilosof, Morand, Krasnov and Nunn2015). To calculate centrality, a bipartite network was projected to a unipartite network, which was subsequently transformed into the network graph object. This was done using the R package ‘tnet’ (Opsahl, Reference Opsahl2009). C was then calculated as eigenvector centrality using the R package ‘igraph’ (Csardi and Nepusz, Reference Csardi and Nepusz2006).
To calculate host contribution to beta diversity (HCBD) of helminth infracommunities, we first transposed each network matrix, so that columns represented helminth species, whereas rows represented individual warthogs. Subsequently, we calculated HCBD values for each individual warthog using function ‘beta.div’ and Hellinger dissimilarity coefficient implemented in the R package ‘adespatial’ (Dray et al., Reference Dray, Bauman, Blanchet, Borcard, Clappe, Guenard, Jombart, Larocque, Legendre, Madi and Wagner2018). In addition, we partitioned total beta diversity of helminth infracommunities into spatial (=between-host) turnover and nestedness components (Baselga, Reference Baselga2010) for the sake of comparison of nestedness component of infracommunity beta diversity between the 2 areas (HNR and KNP). This was done using the function ‘beta.multi’ of the R package ‘betapart” (Baselga et al., Reference Baselga, Orme, Villeger, De Bortoli, Leprieur, Logez, Martinez-Santalla, Martin-Devasa, Gomez-Rodriguez and Crujeiras2023).
Estimation of dark diversity can be done via calculation of the probability of species occurrence based on the co-occurrence of a given species with other species. In this approach, a species that is absent from a given site is considered to be part of the species pool if it usually co-occurs with species present in this site (Ronk et al., Reference Ronk, Szava-Kovats and Pärtel2015; Lewis et al., Reference Lewis, Szava-Kovats and Pärtel2016). For each species, the co-occurrence probability is calculated in the sites where it is actually present and the sites where it is absent. Subsequently, a species is included in dark diversity if it is absent in a site, but its occurrence probability is greater than some threshold (Ronk et al., Reference Ronk, Szava-Kovats and Pärtel2015). Carmona and Pärtel (Reference Carmona and Pärtel2021) presented a method to estimate probabilistic species pools using pairwise co-occurrence data in which species suitability in a site is directly estimated by comparing the realized co-occurrence pattern of each species pair to that expected if there is no association between these species, while the extent of the departure of the observed co-occurrence between the species pair from random association is used as the indicator value for this pair. The best method to calculate the probability of co-occurrence proposed by Carmona and Pärtel (Reference Carmona and Pärtel2021) involved the calculation of the expected number of co-occurrences as the mean value of the hypergeometric distribution (Arita, Reference Arita2016). Here, we used the hypergeometric method and calculated the suitability (=probability to belong or not belong to dark diversity) of each helminth species absent from an individual warthog. The probability of an absent helminth to belong to the dark diversity of an individual warthog is high if its associations with the helminths present in other warthogs are mostly positive, whereas the opposite is true if its associations with the present helminths are negative. We calculated the probabilities of helminths to belong to the dark diversity using the R package ‘DarkDiv’ with the options ‘method = hypergeometric’ and ‘wa = F’ (because numbers of helminths belonging to different species cannot be compared). The helminth dark diversity size for each individual warthog was calculated as the sum of the probabilities of all helminth species absent from this warthog to belong to dark diversity.
To test for the associations between a warthog's sex, age and season of sampling (explanatory variables) and each of the nestedness metrics as well as dark diversity size (response variables), we used generalized linear models (GLMs). In GDMs of d’ and C, the option ‘family = quasibinomial’ was applied because these indices may vary between 0 and 1 and may attain either 0 or 1. In contrast, we applied beta-regression (Cribari-Neto and Zeileis, Reference Cribari-Neto and Zeileis2010) with a logit link function for the analyses involving HCBD because the HCBD value ranges from 0 to 1 without attaining either 0 or 1. Beta-regression approach is the most suitable for models with a response variable of this type because this approach incorporates heteroscedasticity and skewness characteristics for these variables. Beta-regressions were carried out using the R package ‘betareg’ (Cribari-Neto and Zeileis, Reference Cribari-Neto and Zeileis2010). For each metric, we ran a number of models with all possible combinations of the explanatory variables, including an intercept-only model, and then selected the best model based on Akaike information criterion using the R package ‘MuMIn’ (Barton, Reference Barton2020).
Results
Warthogs collected at the 2 localities in South Africa harboured a total of 18 gastrointestinal helminths, comprising 16 species of nematodes, 1 species of cestode and 1 species of trematode (Supplementary Tables S1 and S2). Of these, 17 species were present at KNP, with only Cooperia hungi being absent, whereas 13 species were present at HNR. All 5 species absent from warthogs at HNR (Strongyloides sp., Streptopharagus sp., Trichostrongylus falculatus, Trichuris sp. and Schistosoma sp.), were scarce species at KNP, where they had a prevalence of less than 10% (Supplementary Table S1). Similarly, C. hungi infected a single adult female at HNR. By far the most prevalent species was Probstmayria sp., with a prevalence of 100% at both localities and being the only species present in an adult male at KNP. All warthogs were infected with at least 1 helminth species. While being hosts to a larger pool of species, species richness in warthogs at KNP was lower, with a mean of 5.3 ± 0.2 and a range of 1–9 species per host individual, whereas warthogs at HNR were infected with 5–10 species, with a mean species richness of 7.0 ± 0.2. With the exception of the indirectly transmitted nematodes Physocephalus sexalatus and Streptopharagus sp., the cestode Moniezia sp. and the trematode Schistosoma sp., helminths recovered from warthogs at the 2 localities have a direct life cycle (Supplementary Table S2).
Adults of Murshidia hamata and Murshidia pugnicaudata were amongst the most prevalent and abundant species at both localities, with M. hamata being the dominant of the 2 species, reaching a slightly higher prevalence and abundance than its congener (Supplementary Table S1). Equally prevalent and even more abundant at HNR than Murshidia spp. were adults of Daubneyia mocambiquei and Daubneyia mwanzae. Prevalence of these 4 species ranged between 48–83% at KNP and between 95–100% at HNR. Another common species at HNR with a prevalence of 81% was P. sexalatus, adults of which infected 37% of hosts at KNP (Supplementary Table S1).
Helminths with an intermediate prevalence, i.e. adults infecting less than 50% but more than 10% of hosts at both localities, were Ascaris phacochoeri, I. tuberculata, Trichostrongylus thomasi and Moniezia sp. The remaining species (Strongyloides sp., Streptopharagus sp., C. hungi, Trichostrongylus deflexus, T. falculatus, Trichuris sp. and Schistosoma sp.) had a prevalence of less than 10% (Supplementary Table S1).
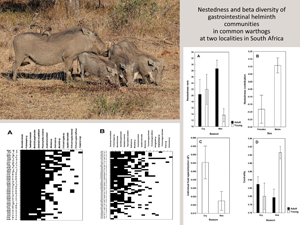
Warthog–helminth networks at the 2 localities are visualized on Fig. 1. Both networks were significantly nested, albeit the degree of nestedness in helminth infracommunities of warthogs from KNP was not especially high (NODFhosts = 73.43, Z = 55.57 for HNR and NODFhosts = 58.90, Z = 50.16 for KNP; P < 0.001 for both). This was further supported by partitioning of beta diversity of helminth infracommunities into species turnover and nestedness components with the nestedness component for warthogs in KNP being substantially lower than that for warthogs in HNR (0.04 vs 0.12, respectively). Summaries of GLM for the effects of a warthog's sex and age as well as season of sampling on nestedness metrics are presented in Table 1. Nestedness rank was higher in adult than in young warthogs, but this was only the case for animals sampled in the wet season in HNR (Fig. 2A). No effect of any explanatory variable on nestedness rank was found in KNP. Nestedness contribution was higher in male than female warthogs (a) independently of their age and season of sampling in HNR (Fig. 2B) and (b) in the wet season in KNP (Fig. 3B), whereas the opposite effect of sex on nestedness contribution was found in KNP in the dry season (Fig. 3B). In HNR, nestedness resultant did not respond to either explanatory variable, whereas in KNP, it demonstrated the same pattern as nestedness contribution in this area, being higher in females in the dry season and higher in males in the wet season (Fig. 3A, B). Individual host specialization (d’) was affected by season of sampling in HNR (higher in the dry season) (Fig. 2C) but by warthog age in KNP (higher in young individuals) (Fig. 3C). Values of centrality were higher in young than adult warthogs sampled in the wet but not the dry season in HNR (Fig. 2D), but no effect of host sex, age or season of sampling on this metric was found in KNP. The latter was also true for IHS in both localities.

Figure 1. Maximally nested presence–absence matrices of individual warthog–helminth associations in Hoedspruit Nature Reserve (A) and Kruger National Park (B). M and F – male and female warthogs, respectively; A and Y – adult and young warthogs, respectively; Dry and Wet – warthogs sampled during dry and wet season, respectively.
Table 1. Coefficients of the best models of the effects of a warthog's sex (SX), age (A), season of sampling (SE) and their interactions on (a) the metrics of nestedness [nestedness rank (NR), nestedness resultant (Nres) and nestedness contribution (Ncont)], (b) indices of the importance of individual warthogs in a warthog–helminth network [individual host specialization (d’), individual host strength (IHS) and centrality (Cent)]; (c) contribution of each individual warthog to beta diversity of helminths in helminth infracommunities (HCBD); and (d) dark diversity size (DDS) of helminths in an individual warthog in Hoedspruit Nature Reserve (HNR) and Kruger National Park (KNP). Reference levels of explanatory variables were female for sex, adult for age and dry season for season. Only significant coefficients are shown. NR, Ncont, d’, Cent and DDS were modelled using generalized linear models (GLM), whereas HCBD was modelled using beta-regression.


Figure 2. Mean (±s.e.) values of nestedness rank (A), nestedness contribution (B), individual host specialization (d’) (C), and centrality (C) in adult and young male and female warthogs sampled during dry and wet seasons from Hoedspruit Nature Reserve.

Figure 3. Mean (±s.e.) values of nestedness resultant (A), nestedness contribution (B), and individual host specialization (d’) (C) in adult and young male and female warthogs sampled during dry and wet seasons from Kruger National Park.
Effects of host-associated variables on community metrics unrelated to nestedness patterns (HCBD and dark diversity size) were revealed in HNR, but not KNP. In particular, contribution to beta diversity of helminth infracommunities differed between warthogs sampled in different seasons, being higher in the dry season in both adult and young female and male warthogs (Fig. 4A). Dark diversity size was higher in adult than young animals independently of sex and season of sampling (Fig. 4B).

Figure 4. Mean (±s.e.) values of host contribution to beta diversity of helminth infracommunities (A) and dark diversity size of these communities (B) in adult and young warthogs sampled during dry and wet seasons from Hoedspruit Nature Reserve.
Discussion
The helminth fauna of warthogs at HNR and KNP was moderately species rich and coincided largely with that of warthogs examined in the Pongola Game Reserve (PGR) in KwaZulu-Natal Province, South Africa (Junker et al., Reference Junker, Spickett, Swanepoel, Krasnov, Boomker and Hoffman2019), at various localities in Limpopo Province (van Wyk and Boomker, Reference van Wyk and Boomker2011) and Namibia (Horak et al., Reference Horak, Biggs, Hanssen and Hanssen1983). Gastrointestinal helminth species that have consistently been found in warthogs at several localities in southern Africa and form the core of parasite assemblages of this host are the nematodes A. phacochoeri, D. mocambiquei, D. mwanzae, I. tuberculata, M. hamata, M. pugnicaudata, P. sexalatus and Probstmayria sp. as well as cestodes of the genus Moniezia (Horak et al., Reference Horak, Biggs, Hanssen and Hanssen1983; van Wyk and Boomker, Reference van Wyk and Boomker2011; Junker et al., Reference Junker, Spickett, Swanepoel, Krasnov, Boomker and Hoffman2019). The gap between helminth species present in infracommunities at a given locality and those that are known parasites of warthogs, i.e. dark diversity, is likely influenced by local environmental conditions that enable the survival of free-living stages and intermediate hosts (Junker et al., Reference Junker, Spickett, Swanepoel, Krasnov, Boomker and Hoffman2019). Furthermore, varying sets of sympatric hosts might add to the available pool of helminth species at a given locality (Huang et al., Reference Huang, Bininda Emonds, Stephens, Gittleman and Altizer2014; Junker et al., Reference Junker, Boomker, Horak and Krasnov2022). It is noteworthy, that warthog–helminth networks at HNR and KNP were more species rich than those at other localities. In addition, at HNR, dark diversity was higher in adult than young warthogs, possibly because of a better immune response to parasite infection in adults (Fellis et al., Reference Fellis, Negovetich, Esch, Horak and Boomker2003; Tinsley et al., Reference Tinsley, Stott, York, Everard, Chapple, Jackson, Viney and Tinsley2012; Nielsen et al., Reference Nielsen, Morrill, Skírnisson, Stenkewitz, Pálsdóttir and Forbes2020). In support of this, the prevalence of Moniezia sp. at HNR was 76.2% in young warthogs, but dropped to 5% in adults, which typically acquire immunity after the first infection (Reinecke, Reference Reinecke1983).
Nestedness
Warthog-parasite networks at both localities followed a nested pattern, although less pronounced in KNP than HNR. Our analyses demonstrated the influence of both host sex and age, as well as season of sampling on the structure and dynamics within these networks. Interestingly, and as shown in previous studies (Zelmer et al., Reference Zelmer, Paredes-Calderón, León-Règagnon and García-Prieto2004; Chaisiri et al., Reference Chaisiri, Chou, Siew, Morand and Ribas2017; Spickett et al., Reference Spickett, van der Mescht, Junker, Krasnov, Haukisalmi and Matthee2019; Junker et al., Reference Junker, Boomker, Horak and Krasnov2022), the effect of a given factor on the same metric can vary between localities.
At HNR, nestedness rank was significantly higher in adult than young warthogs in the wet season. A number of studies have shown parasite infracommunities of adult hosts to be more species rich when compared to young animals. They associated this with an increase in age and size leading to higher mobility and/or food intake, which in turn increases exposure to free-living infective stages or intermediate hosts of parasites (Guégan and Hugueny, Reference Guégan and Hugueny1994; Rohde et al., Reference Rohde, Worthen, Heap, Hugueny and Guégan1998; Zelmer et al., Reference Zelmer, Paredes-Calderón, León-Règagnon and García-Prieto2004). Furthermore, females of ruminants have been shown to be more susceptible to parasite infection during the periparturient and lactating period due to a loss of immunity (Barger, Reference Barger1993; Beasley et al., Reference Beasley, Kahn and Windon2010). In warthogs, this period extends from November to February (Somers et al., Reference Somers, Rasa and Penzhorn1995), and could thus add to the above phenomenon. On the other hand, adult male warthogs spend much time defending females rather than feeding during the mating season from May to June (Somers et al., Reference Somers, Rasa and Penzhorn1995), which coincides with the dry season (May to August).
Male warthogs contributed more to nestedness than females at HNR. Warthog males are larger than females, offering larger niche space for parasites, have larger home ranges, and disperse more than females, including roaming between females during mating season (Somers et al., Reference Somers, Penzhorn and Rasa1994). Consequently, males have an increased chance of acquiring diverse parasite infracommunities, which contributes to nestedness (Zelmer et al., Reference Zelmer, Paredes-Calderón, León-Règagnon and García-Prieto2004; González and Poulin, Reference González and Poulin2005; Cardoso et al., Reference Cardoso, Andreazzi, Maldonado Junior and Gentile2021).
In the KNP, both host sex and season influenced nestedness contribution. In the wet season, the pattern was similar to that seen at HNR, but was converse in the dry season, when values for nestedness contribution were higher in females than males. As mentioned above, adult male warthogs foregoing foraging in the interest of defending a possible mate might reduce exposure to parasites.
Values of nestedness resultant where higher in females than males in KNP during the dry season, but higher in males than females during the wet season. This follows the same pattern as nestedness contribution and is likely due to the same physiological and behavioural gender differences as highlighted above. Environmental gradients between the 2 localities might mediate the extent to which host heterogeneity influences parasite transmission in relation to host sex and age classes of warthogs (Spickett et al., Reference Spickett, van der Mescht, Junker, Krasnov, Haukisalmi and Matthee2019; Junker et al., Reference Junker, Boomker, Horak and Krasnov2022).
Host specialization and centrality
At HNR, values of individual host specialization (d’) were higher in the dry than the wet season; indicating that the distribution of helminth species in the dry season was patchier than in the wet season. The observed differences likely reflect varying tolerance limits of different helminth species towards environmental stressors such as temperature and moisture (Rossanigo and Gruner, Reference Rossanigo and Gruner1995; O'Connor et al., Reference O'Connor, Walkden-Brown and Kahn2006; Costa-Neto et al., Reference Costa-Neto, Cardoso, Boullosa, Maldonado and Gentile2019), allowing only those with wider tolerance ranges to persist during the dry season. The reduced risk of desiccation during the wet season especially profits helminth species with direct life cycles whose infective stages are exposed to the external environment and which comprise the majority of species in infracommunities in warthogs.
At KNP, on the other hand, d’ values were significantly higher in young than adult warthogs, suggesting that helminth species with poor colonization ability might depend on young animals with naïve immune systems.
Interaction between age and season led to significantly higher values of centrality in young when compared to adult warthogs at HNR in the wet season. A converse effect of age on helminth–parasite networks was found in nyalas in Mkhuze, where values of centrality were higher in adult than young females, and in iMfolozi, values of centrality were higher in male than female nyalas irrespective of the age (Junker et al., Reference Junker, Boomker, Horak and Krasnov2022). Such network variation is likely caused by differences in host mobility, foraging preferences, immunity or sociality between the sexes or associated with an increase in age and influenced by habitat heterogeneity and climate conditions at a given locality (Fellis et al., Reference Fellis, Negovetich, Esch, Horak and Boomker2003; Zelmer et al., Reference Zelmer, Paredes-Calderón, León-Règagnon and García-Prieto2004; Foata et al., Reference Foata, Mouillot, Culioli and Marchand2006; Negovetich et al., Reference Negovetich, Fellis, Esch, Horak and Boomker2006; Saeed et al., Reference Saeed, Maddox-Hyttel, Monrad and Kapel2006; Bellay et al., Reference Bellay, Oda, Almeida-Neto, de Oliveira, Takemoto and Balbuena2020).
Young warthogs at HNR contributed more to parasite transmission in the network than adults. Piglets form part of matriarchal groups and subsequently reorganise into yearling groups from which younger males move on to bachelor groups. In contrast, adult males are typically solitary and females, while organized in matriarchal groups during much of the year, leave the group during the farrowing season (Somers et al., Reference Somers, Rasa and Penzhorn1995). High host population density is known to increase the probability of contact between the infective stages of a parasite and its host (Arneberg et al., Reference Arneberg, Skorping, Grenfell and Read1998; Arneberg, Reference Arneberg2002; Cardoso et al., Reference Cardoso, Andreazzi, Maldonado Junior and Gentile2021). Their higher sociality, combined with changing group associations and related habitat shifts, likely expose young warthogs to a larger variety of helminth taxa when compared to adults.
Beta diversity
Irrespective of their age or sex, warthogs sampled during the dry season at HNR contributed more to beta diversity of infracommunities than those sampled during the wet season. A reduced and uneven spread of infective stages in the environment during the dry season, resulting from increased selective pressure of adverse climatic conditions (Brooker et al., Reference Brooker, Clements and Bundy2006), might explain this.
In general, the effects of sex, age and season were more pronounced in HNR than in KNP, but they also often varied at the 2 localities. While all sampling sites of warthogs were located in the Granite Lowveld, local habitat differences associated with variations in the potential intermediate and final host fauna of parasites would have been more pronounced between sampling sites in HNR and KNP than within HNR and KNP themselves and would have affected transmission dynamics at the 2 localities.
In conclusion, the present study suggests that helminth communities of warthogs are structured networks that are influenced by numerous internal factors, such as host sex and age as well as the dietary and/or behavioural patterns associated with these. Furthermore, external factors, such as climate, habitat diversity and co-occurring host species at a given locality contribute to network organization. Varying combinations of these can result in a multitude of responses shaping each host–parasite network individually. It is therefore only with great circumspection that network patterns observed at 1 locality should be applied to the same host–parasite system in a different geographic setting.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182023000719.
Data availability
All data generated or analysed during this study are included in this published article. The datasets used and/or analysed are available from the corresponding author upon reasonable request.
Authors’ contributions
K. J. and B. R. K. conceived the study. I. G. H. and J. B. collected the material and identified the helminths. B. R. K. analysed the data. K. J. and B. R. K. drafted the manuscript. All authors participated in finalizing the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed.