The WHO defines Fe deficiency (ID) as serum ferritin under 12 µg/l(1). ID is one of the most common micronutrient deficiencies worldwide and is a main cause of anaemia along with infections and parasitic infestations(1–Reference Akkermans, van der Horst-Graat and Eussen3). In 2015, ID was reported in Western Europe, with a prevalence of 11·8 % in a study of 12- to 36-month-old children from Germany, the Netherlands and the UK(Reference Akkermans, van der Horst-Graat and Eussen3).
ID affects myelination and basal ganglia function and is associated with impaired neurodevelopment(Reference Bakoyiannis, Gkioka and Daskalopoulou4–Reference Bruner, Joffe and Duggan8). ID has been associated with delayed cognitive development during the rapid growth spurt of the brain in early childhood(Reference Lozoff, Beard and Connor6,Reference Jauregui-Lobera7) , leading to potential longitudinal effects on cognition later in life, which are exemplified by study findings and positive effects of Fe supplementation for non-anaemic ID on cognition, learning capacity and memory(Reference Lukowski, Koss and Burden5,Reference Lozoff, Beard and Connor6,Reference Bruner, Joffe and Duggan8,Reference Abdullah, Kendzerska and Shah9) .
ID may also increase the risk of infection as Fe is required for normal immune function as a critical component of peroxide- and nitrous oxide-generating cellular enzymes used in the bactericidal activity of macrophages and because Fe affects T-cell number and function(Reference Jonker and Hensbroek10,Reference Beard11,Reference Michaelsen, Milman and Samuelson12) .
Infant’s need for Fe is greater than that of an adult because of its rapid growth and development(Reference Michaelsen, Milman and Samuelson12,Reference Domellof, Braegger and Campoy13,Reference Gondolf, Tetens and Michaelsen14) . A negative association was found between knee-heel-growth velocity and serum ferritin levels over the age of 2–6 months among healthy Danish infants, indicating that Fe stores are used during growth(Reference Michaelsen, Milman and Samuelson12). The size of Fe stores at birth depends on several factors. Preterm infants have lower Fe stores as Fe is mainly transferred from the maternal circulation to the fetus after 30 weeks of gestation, while delayed cord-clamping postpartum has a positive effect on Fe stores in full-term infants(Reference Bakoyiannis, Gkioka and Daskalopoulou4,Reference Oliveira Fde, Assis and Martins15) .
Regardless of the size of Fe stores at birth, they are depleted during approximately the first 6 months of life(Reference McLean, Cogswell and Egli2,Reference Akkermans, van der Horst-Graat and Eussen3,Reference Domellof, Braegger and Campoy13) . Dietary Fe intake is thus needed from 4 to 6 months onwards as Fe absorption from infant formula is poor and Fe content in human milk is very low (0·2–0·9 mg/l)(Reference Mastroeni, Okada and Rondo16–Reference Shashiraj, Faridi and Singh18). Fe or Hb status of healthy mothers does not correlate with Fe content in their breast milk 6 months postpartum, so increasing the Fe intake in the diet of a healthy mother will not increase the Fe content of her milk(Reference Mastroeni, Okada and Rondo16–Reference Shashiraj, Faridi and Singh18).
Dietary Fe is either haem Fe from myoglobin and Hb from meats and fish, or non-haem Fe from vegetable sources. The bioavailability of haem Fe is generally better than that of non-haem Fe. Furthermore, the composition of the diet is important as, for example, vitamin C and haem Fe enhance absorption of non-haem Fe, whereas Ca in cows’ milk may inhibit Fe absorption(Reference Teucher, Olivares and Cori19,Reference Hallberg, Rossander-Hultén and Brune20) .
Little is known about the prevalence of ID and its associated early-life factors in Scandinavian 18-month-old children. This population is significant for investigating possible longitudinal associations between ID and early-life factors and recommendations on Fe supplementation in the first year of life. A study among Danish 9-month-olds found that ferritin levels were positively associated with compliance to oral Fe supplementation or daily intake of ≥400 ml Fe-fortified infant formula from 6 months of age and that intake of meat and fish was not significantly associated with ferritin levels(Reference Gondolf, Tetens and Michaelsen14). Until 2015, the Danish recommendations were that full-term infants should receive Fe supplementation (about 8 mg/d) or at least 400 ml Fe-fortified formula or gruel from age 6 to 12 months. Since 2015 oral Fe supplementation is no longer recommended. Parents are now recommended not to offer cows’ milk, but only breast milk or infant formula throughout the first year of life, and they are recommended to serve Fe-rich food such as fish and meat from 6 months of age(Reference Gondolf, Tetens and Michaelsen14,21) . However, in Denmark only 60 % of children are exclusively breast-fed until 12 weeks of age and only 12 % of children are exclusively breast-fed until 6 months of age, meaning that most children are introduced to solids before 6 months of age(Reference Hörnell, Lagström and Lande22,Reference Bruun, Buhl and Husby23) . In this cohort of children born in 2010–2013, we, therefore, have the possibility to examine the importance of Fe supplementation within the first year of life for Fe status in later childhood. The aims of the present study were to determine the prevalence of ID and to describe factors associated with ID longitudinally in healthy 18-month-old Danish children.
Materials and methods
The present study is a subproject of the Odense Child Cohort (OCC), a birth cohort of 2550 children enrolled during the years 2010–2013 from the municipality of Odense, Denmark. Blood samples, anthropometric and clinical measures as well as questionnaire data on, for example, supplementation, diet and breast-feeding, were collected at predetermined ages. Further details on the OCC are previously published(Reference Kyhl, Jensen and Barington24). The present study uses data collected at birth and at 18 months of age.
The blood samples we used from the OCC had been randomly selected and analysed for plasma ferritin and C-reactive protein (CRP) in a separate study of normal reference levels of ferritin in healthy children. The present study was conducted by the Department of Clinical Biochemistry and Pharmacology (CBP) at the Odense University Hospital and it is referred to as the CBP study, which is not yet published.
Blood samples
Samples of venous blood were collected at Hans Christian Andersen Children’s Hospital, Odense University Hospital, by trained paediatric phlebotomists. Non-fasting venous blood samples were collected between 08.00 and 17.00 hours. Samples for values of ferritin and CRP were collected in BD Vacutainer® PST™ Gel Separator Tubes with Li heparin. They were kept at room temperature, centrifuged and analysed within 4 h of collection.
Ferritin values were analysed by chemiluminescence microparticle immunoanalysis technology (Ferritin 7K59; Abbott Laboratories) using Architect Instruments (Abbott Laboratories). CRP concentrations were measured by immunoturbidimetric assay (CRP Vario 6K26; Abbott Laboratories) using Architect Instruments (Abbott Laboratories). The inter-assay CV were <6 % for ferritin and <3 % for CRP.
As ferritin is an acute-phase reactant, children with CRP levels above 10 mg/l were excluded from the study. In addition, children born prematurely or twins, children who had contact with the paediatrics department at the Odense University Hospital for infection within 7 d before or after blood sampling and had chronic disease were excluded from the study.
Questionnaire data
The questionnaires used in the Danish National Birth Cohort(Reference Olsen, Melbye and Olsen25) were modified and used for this study. The present study used parent-reported background data such as birth date, birth weight and birth length, socio-economic status of parents, etc. from time of enrolment as well as parent-reported questionnaire data at 18 months of age on breast-feeding, Fe supplementation from 6 to 12 months, infant formula and current intake of cows’ milk, meat and fish.
Parents reported the number of weeks the child had been exclusively breast-fed (defined as only breast milk and water) and partially breast-fed (defined as any breast milk concurrent with other foods or drinks besides water), the child’s age when breast-feeding ceased, the age at which the child was fed infant formula for the first time and the brand that was used and whether the child was still fed infant formula. However, data on the volume of infant formula were not obtained, thus excluding the possibility of analysing the intake of infant formula. Exclusive breast-feeding beyond 4 months of age was analysed as a binary variable with a cut-off at 17 weeks, since this is a common practice among breast-feeding parents in Denmark, even though national guidelines recommend exclusive breast-feeding for 6 months(Reference Hörnell, Lagström and Lande22).
Parents also reported whether they chose to give their children the recommended 8 mg/d of oral Fe supplementation and the age at which supplementation was started and stopped. The most common form of supplementation was Fe drops(Reference Gondolf, Tetens and Michaelsen14). Only children receiving 8 mg/d of oral Fe supplementation continuously in the period from 6 to 12 months of age were allocated to the ‘yes’ group of oral Fe supplementation. Children who did not receive oral Fe supplementation continuously in this period were allocated to the ‘no’ group of Fe supplementation.
The average intake of fish and meat per d, week or month was reported as children’s spoonful (equivalent to 15 g), half a Danish meatball or one slice of meat or fish on bread. Parents were given the choice to report ‘My child is almost never served meat/fish’. Current intake of unmodified cows’ milk, both as drinks and as yogurt, was reported in units of 100 ml/d or ‘uncertain because of the intake in day care’ or ‘does not tolerate cows’ milk’. Ca and Fe were not quantified using the questionnaire, as the variety of different foods in these categories would make such an estimation inaccurate. Instead we only focused on the overall intake of fish, meats and dairy products.
The child’s weight was measured in kg. The child’s length was measured as crown–heel length in cm at 18 months. Weight velocity (kg/month) and length velocity (cm/month) were measured from time of birth to exact age at measurement. For children aged 0–5 years, the z scores of weight and length for age were calculated using the WHO growth standards(26). Anthropometric measurements from birth were collected routinely by midwives at birth, whereas measurements at 18 months of age were collected by four technicians with a minimum of 10 years’ experience in paediatrics at the OCC(Reference Kyhl, Jensen and Barington24).
Statistical analyses
A power calculation showed that the 370 samples in the present study provided a sufficient sample size to determine the prevalence of ID at a confidence level of ± 3 %, assuming a known prevalence of ID in Western Europe of 11·8 %(Reference Akkermans, van der Horst-Graat and Eussen3). P values <0·05 were thus considered significant.
ID was defined as a binary outcome at the plasma ferritin cut-off value <12 µg/l. Baseline differences between the two groups, ID and non-ID, were tested by independent t tests.
The association between ID and our two main variables (exclusive breast-feeding beyond 4 months and intake of oral Fe supplementation) was analysed using both crude and adjusted logistic regression models. The crude model only tested the direct association between each of the main exposures individually to the outcome. The adjusted model included three variables that were chosen after stepwise backward elimination analyses with the elimination cut-off value P > 0·20 (i.e. current intake of unmodified cows’ milk/yogurt, fish and meat) as well as three clinically relevant, albeit non-significant, variables (duration of partial breast-feeding, maternal education and sex) and basic characteristics and anthropometric measures (see Tables 2 and 3).
Table 1. Basic characteristics of all participants in the Odense Child Cohort (OCC), excluded participants and the final study participants
(Numbers of participants and percentages)
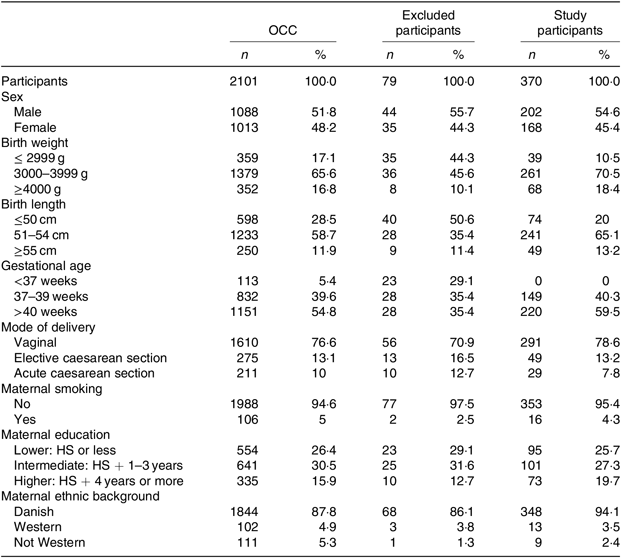
HS, high school.
Table 2. Basic characteristics of the 370 study participants grouped in non-iron-deficient (non-ID) and iron-deficient (ID) participants
(Numbers of participants and percentages)
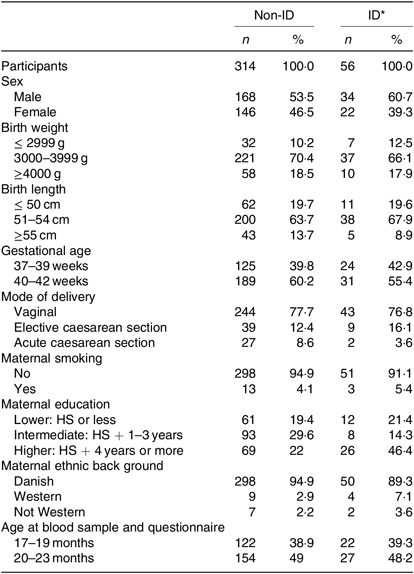
HS, high school.
* ID defined by plasma ferritin <12 μg/l.
Table 3. Anthropometric characteristics of the 370 study participants grouped in non-iron-deficient (non-ID) and iron-deficient (ID) participants
(Mean values and standard deviations)
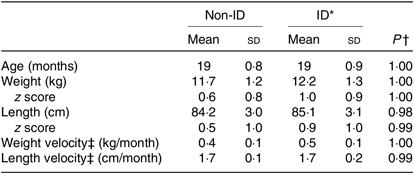
* ID defined by plasma ferritin <12 μg/l.
† Independent t tests between non-ID and ID values.
‡ Velocity, monthly average from birth to exact age.
The final adjusted logistic regression model was validated in a nested likelihood ratio test against the crude logistic model with each explanatory variable in turn. The adjusted logistic regression model showed better goodness of fit than the crude model.
To deal with the missing values of the explanatory variables, the crude models were tested in a smaller sample consisting of complete cases and thus not influenced by missing values of the explanatory variables. Missing values for duration of exclusive breast-feeding were assumed to be missing at random, so we used the iterative chained equations approach. The adjusted regression model was repeated with multiple imputations on exclusive breast-feeding to test the impact of missing values on our adjusted model. The findings with multiple imputations did not differ from the findings of the adjusted regression model and are thus not discussed here. All statistical analyses were done in STATA 14.1 statistical software (StataCorp LP).
Ethics
The OCC and the normal-reference study at CBP were approved by the Regional Scientific Ethical Committees for Southern Denmark under the project IDs S-20090130 and S-20130159, respectively. Both projects are approved by the Danish Data Protection Agency. Written consent was collected from all parents participating in the OCC. The Regional Scientific Ethical Committees of Southern Denmark defined the present study as a database study not requiring further approval because we used data from blood samples already obtained and analysed by the OCC and the CBP study.
Results
Of the 2550 children enrolled in the OCC, blood samples were taken from 1150 children at their 18-month-old examination. Most of the missing blood samples were due to either the parents declining, or the child crying or moving too much, hindering the collection of blood samples. Some venepunctures were unsuccessful, and the phlebotomists routinely only punctured a child once. Of the 1150 blood samples collected, the CBP randomly included 449 samples for their study on normal reference levels in healthy children, while seventy-nine were excluded due to various reasons (Fig. 1).
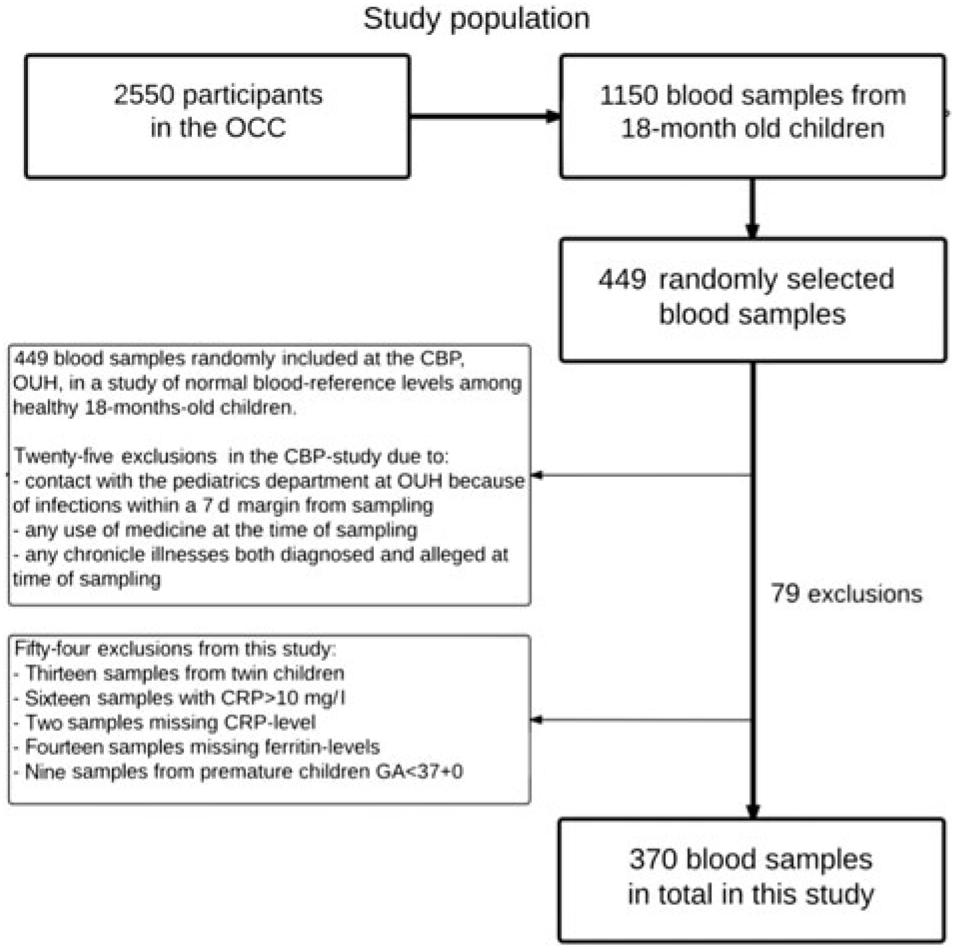
Fig. 1. Flow chart of study population. OCC, Odense Child Cohort; CBP, Clinical Biochemistry and Pharmacology; OUH, Odense University Hospital; CRP, C-reactive protein; GA, gestational age.
The final study population of 370 participants was similar to the excluded OCC participants with regard to sex, mode of delivery, maternal smoking, educational level and ethnic background. Both groups had a slight overweight of males, the majority were delivered by vaginal birth, the vast majority of mothers were non-smokers and Danish by ethnicity (Table 1). The participants in our study did differ from the general OCC with regard to birth weight, birth length and gestational age as a natural consequence of excluding premature children, twins and children with chronic diseases (Table 1). With regard to all background data, no significant differences were found between the ID and non-ID groups. There were low numbers of missing data, and they were evenly distributed between groups. Potential interactions between all variables were tested individually and found non-significant.
Iron deficiency prevalence
The overall prevalence of ID in our study population was 15·1 %, as fifty-six out of 370 children had ferritin values <12 µg/l. Only one child in our study had ID anaemia. Children with ID did not differ from non-ID children with regard to sex, gestational age, birth weight and length, mode of delivery, exact age at blood sampling, maternal smoking or ethnicity. More mothers in the ID group were highly educated (Table 2), but this was a non-significant difference as the OR for ID in children of highly educated mothers was 1·92 (95 % CI 0·62, 5·92) compared with intermediate and low educated mothers.
Anthropometrics
Children in the ID and non-ID groups were not significantly different in absolute weight/length, weight/length velocity or weight/length z scores. For 0 to 5-year-old children, all z scores were within the normal range of WHO growth standards (Table 3).
Factors associated with iron deficiency
Exclusive breast-feeding for longer than 4 months was significantly associated with ID (OR 5·97; 95 % CI 1·63, 21·86), while no intake of oral Fe supplementation from the age of 6–12 months was also significantly associated with ID at 18 months of age (OD 3·99, 95 % CI 1·33, 11·97; Table 4).
Table 4. Risk for iron deficiency (ID; plasma ferritin <12 µg/l)
(Odds ratios and 95 % confidence intervals)
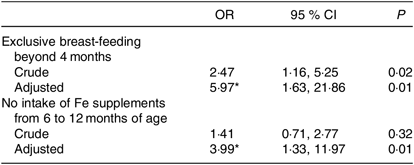
* Adjusted for sex, maternal education, duration of partial breast-feeding and current intake of milk, fish and meat. None of these was significantly associated with ID.
The following variables were not significantly associated with ID: sex of the child (boy OR 0·80, 95 % CI 0·29, 2·18), duration of partial breast-feeding (measured continuously per week, OR 1·00, 95 % CI 0·97, 1·04), current intake of milk or other dairy products (over 500 ml/d, OR 0·82, 95 % CI 0·27, 2·50), fish intake (in g/week, OR 1·00, 95 % CI 0·99, 1·01) and meat intake (in g/week, OR 1·00, 95 % CI 1·00, 1·00).
Discussion
Iron deficiency prevalence
In the present study of 18-month-old Danish children, we found an ID prevalence of 15·1 %. This is in line with studies of children in Germany, the Netherlands and the UK, where the ID prevalence was on average 11·8 %(Reference Akkermans, van der Horst-Graat and Eussen3). It is also comparable to studies of 18- to 36-month-old children in Northern Israel with an ID prevalence of 19·9 %(Reference Levin, Harpaz and Muklashi27) and several American studies on toddlers, where the ID prevalence varied between 7 and 20·5 %(Reference Edena and Sandoval28).
Until 2015, the recommendation in Denmark was that infants aged 6–12 months should receive either 8 mg Fe as a daily supplement or at least 400 ml of Fe-fortified formula daily(Reference Gondolf, Tetens and Michaelsen14). This recommendation was valid during our study period. In 2015, new recommendations similar to most other Western countries were published by the Danish health authorities in accordance with the 2014 European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) position paper on Fe requirements of infants and toddlers(Reference Domellof, Braegger and Campoy13,21) . Infants aged 6–12 months with normal birth weight and born at full-term are no longer recommended Fe supplements. Parents are instead encouraged to offer Fe-rich food such as meat and fish from 6 months of age, advised not to offer unmodified cows’ milk and yogurt before the age of 12 months and thereafter to limit the intake of unmodified cows’ milk to under 500 ml/d(21).
Oral iron supplementation
We found a significant association between no oral Fe supplementation between 6 and 12 months of age and an increased risk of ID at 18 months of age. Our results are similar to those of Gondolf et al. who found that Fe supplementation in the 9-month-olds (either as drops or fortified formula) was associated with higher ferritin levels, while meat and fish intake was not associated with ferritin levels(Reference Gondolf, Tetens and Michaelsen14). The key difference from our study is that the study by Gondolf et al. was carried out while the children were still at the age of receiving Fe supplementation. Our finding of an association between no Fe supplementation from 6 to 12 months of age and higher risk for ID at 18 months of age (6 months after Fe supplementation ceased) indicates not only an immediate association between supplementation and ferritin levels but also a long-term association.
Our findings may stand in contrast to the new Danish recommendations of no Fe supplementation for children born at full term(21). However, as studies have found association between higher risk of ID and the intake of unmodified cows’ milk(Reference Akkermans, van der Horst-Graat and Eussen3), which inhibits Fe absorption and contains very little Fe in itself, the useful recommendations are avoiding the intake of unmodified cows’ milk before 12 months of age and encouraging the intake of Fe -rich foods from 6 months of age.
Furthermore, it is important to remember that oral Fe supplementation of Fe-sufficient children may have adverse effects as found in one Swedish study where Fe-sufficient children who received oral Fe supplementation had reduced growth in length(Reference Dewey, Domellof and Cohen29). This is a relevant consideration as the vast majority of our study population was Fe sufficient.
It would be relevant to conduct a new study among 18-month-old Danish children to evaluate whether the new recommendations have had any influence on the ID prevalence at this age.
Exclusive breast-feeding beyond 4 months
We found an association between exclusive breast-feeding beyond 4 months and increased risk of ID at 18 months of age. This is in line with other studies(Reference Akkermans, van der Horst-Graat and Eussen3,Reference Domellof, Braegger and Campoy13) , and the American Academy of Paediatrics has suggested that all exclusively breast-fed infants should receive Fe supplements of 1 mg/kg/d from 4 months of age (and even if they are only partially breast-fed from 4 months but receive more than half of their daily feeds as human milk). Our findings may support this recommendation, and it may be relevant to examine whether Fe supplementation from 4 to 6 months of age would be an attractive alternative to introducing Fe-rich solids at 4–6 months of age. A systematic review from 2013 on Nordic children concluded that exclusive breast-feeding beyond 4 months is associated with better outcomes, for example, less overweight and fewer infections(Reference Hörnell, Lagström and Lande22).
Current diet
We found no association between current diet and ID at 18 months of age. This is in accordance with the study of 9-month-old children by Gondolf et al. (Reference Gondolf, Tetens and Michaelsen14), indicating that current diet is not significantly associated with Fe status. However, both studies were conducted when Fe-rich food such as fish and meat from 6 months of age were not emphasised in food recommendations and unmodified cows’ milk was offered before 12 months of age.
We also have no information on cord-clamping at birth or intake of fruit, vegetables and legumes, all of which could influence the child’s current Fe status(Reference Gondolf, Tetens and Michaelsen14,Reference Oliveira Fde, Assis and Martins15,Reference Teucher, Olivares and Cori19,Reference Hallberg, Rossander-Hultén and Brune20) . Future studies of dietary factors associated with ID in Scandinavian toddlers should include these aspects.
Growth
We did not find associations between anthropometric measurements and the risk for ID. A previous Danish study found negative associations between knee–heel growth velocity and serum ferritin levels in younger infants from 2 to 6 months(Reference Michaelsen, Milman and Samuelson12). We used crown–heel length for our length measurements.
Educational level of mothers
This factor seemed to be associated with ID as the proportion of highly educated mothers in the ID group was more than twice compared with the non-ID group. However, education was not significantly associated with ID after adjustment for breast-feeding. The initially observed difference in ID prevalence among children of highly educated mothers v. those of intermediate and low education was not controlled for breast-feeding, and the proportion of mothers who breast-fed exclusively for more than 4 months was 58·9 % (43/73) among highly educated women, 42·6 % (43/101) among mothers with intermediate education and 41·1 % (39/95) among mothers with low education. The explanatory factor is thus likely to be the duration of exclusive breast-feeding beyond 4 months rather than educational level per se.
The OCC consists of more highly educated, predominantly ethnic Danish non-smoking parents compared with the general Danish and Scandinavian populations(Reference Bruun, Buhl and Husby23). The prevalence of ID has been linked to lower socio-economic groups, for example, in India(Reference Bharati, Pal and Chakrabarty30). In the present study, the higher proportion of children with ID in the group of highly educated mothers may be explained by the strong association between exclusive breast-feeding above 4 months and the increased risk of ID. More of the highly educated mothers breast-fed exclusively beyond 4 months compared with mothers with intermediate and lower education. Therefore, there is a risk of overrepresentation of ID in the OCC in comparison with the Danish population even though our prevalence data are in line with other studies from similar countries.
Questionnaire data were collected at 18 months of age and thus up to 1 year after the actual exposure (e.g. the duration of exclusive breast-feeding and the start of Fe supplementation). This leads to less accurate data recordings and possibly weaker associations. However, we have no reason to believe that these inaccuracies caused systematic biases in the reporting of Fe status as parents were unaware of the outcome (ID or non-ID).
Ferritin can be measured either in plasma collected in Li–heparin tubes, as in the present study, or in serum collected in serum separator tubes. However, a clinical biochemistry study on the stability of routine tests found that only ferritin showed a minor bias in results after 10 h of storage, when comparing samples from Li–heparin tubes with those from serum tubes and using similar analytical methods as in our study. Even with this minor bias, the study concludes that whole blood in either Li–heparin or serum tubes stored for 10 h at 20–22°C can be used for routine analysis without restrictions(Reference Henriksen, Faber and Moller31). The blood samples in our study were analysed within 4 h of collection, so we assume that the plasma ferritin values and ID prevalence are comparable to those of other studies that measure serum ferritin.
Conclusion
The prevalence of ID among healthy Danish 18-month-old children was 15·1 %. We found a higher risk of ID among children exclusively breast-fed beyond 4 months of age and among children with no Fe supplementation from 6 to 12 months of age. We found no association between ID and maternal educational level, duration of partial breast-feeding or the child’s current intake of milk, fish and meat.
Further prevalence studies should determine whether the new Danish recommendations of no Fe supplements and encouragement of Fe-rich complementary foods increase the prevalence of ID among healthy full-term children. If this is the case, randomised controlled trials are needed to investigate the efficacy, safety and long-term effects of oral Fe supplementation in infancy for prevention of ID in Scandinavian children. Further studies exploring dietary factors that contribute to healthy Fe status among Scandinavian children are also needed.
Acknowledgements
The authors thank Claire Gudex, Department of Clinical Research, University of Southern Denmark, for proofreading the manuscript.
The present study was funded by the Ville Heise Grant (grant no./Jr.nr.: M10-15), which had no role in the design, analysis or writing of this article.
A. T. N. A. is the main author of this article and carried out the study. S. H. is the senior investigator of the Odense Child Cohort, who collected all the data used in the present study. He participated in the design of the study, its research questions and in the interpretation of the analyses and also edited the manuscript. C. M. participated in the design of the study, its research questions and in the interpretation of the analyses and also edited the manuscript. S. D. S. participated in the design of the study, its research questions, in data analysis and in the interpretation of the analyses and also edited the manuscript. H. B. K. participated in the sorting of data and in the methodical execution of the study. M. B. S. analysed the blood samples and participated in the writing of the methodological section of this article. All authors approved the final manuscript.
The authors declare no conflicts of interest.










