Introduction
Tuberculosis (TB) is a chronic communicable disease caused by Mycobacterium tuberculosis. The WHO estimates that in 2018, 10 million people were affected by TB. Especially, the number of TB cases is larger in South-East Asia (44%), Africa (24%) and the Western Pacific (18%) [1]. Among single infectious pathogen illnesses, a high proportion of deaths results from TB, which remains a threatening public health issue worldwide [2, Reference Furin, Cox and Pai3]. As a recommended anti-TB strategy, short-course first-line medication regimens have been proved to be effective [1]. Isoniazid is of great importance to kill bacilli during the early anti-TB period. With the addition of pyrazinamide, the sterilizing effect gets better [Reference Blumberg4, Reference Gumbo5]. Rifampicin plays a pivotal role in declining non-replicating bacteria and reduces the TB relapse rate [Reference Liu6]. However, drug-induced liver injury (DILI) associated with the above medications is a common adverse drug reaction. Hepatotoxicity is likely to develop further severely when used in combination, especially on the addition of pyrazinamide [Reference Sharma7–Reference Lin9]. Thus, anti-TB DILI would result in the interruption of chemotherapy regimens and even cause death.
Without novel agents identified to replace first-line drugs, risk factors should be taken into consideration when preventing the occurrence of DILI. The regions with high endemicity for TB largely overlap that with a high hepatitis B virus (HBV) prevalence, especially in Asia and Africa [Reference Schweitzer10]. Many epidemiological studies were conducted to investigate whether HBV infection can increase the incidence and severity of anti-TB DILI, but the results remain inconsistent [Reference Isa11, Reference Kim12]. Recently, a meta-analysis reported that articles have indicated that HBV infection may increase the risk of DILI in the therapy for active TB [Reference Wang13]. However, articles included in the study were only published in English language, and multiple subgroup analyses were not carried out to explore the clinical variability. Given the high burden of TB and HBV infection, the situation of China attracts much attention. In China, most researches conducted by the front-line physician on the subject are published in Chinese. Therefore, the previous meta-analysis results may be subject to potential publication bias. We will perform a meta-analysis to estimate whether HBV infection would increase the risk of DILI during first-line TB treatment based on cohort studies. Furthermore, subgroup analyses were conducted to identify the sources of heterogeneity.
Methods
The present meta-analysis was conducted in accordance with the PRISMA 2015 guideline [Reference Shamseer14].
Literature search strategy
To search for the cohort studies of HBV infection in relation to DILI risk during TB treatment, a literature search was conducted on 20 June 2019 and updated on 23 February 2020 in PubMed, Web of Science and China National Knowledge Infrastructure. The following medical subject heading terms were used: ‘anti-tuberculosis’ OR ‘tuberculosis treatment’ OR ‘TB treatment’ AND ‘chronic hepatitis B’ OR ‘hepatitis B virus’ OR ‘HBV’. Additional articles were retrieved by a manual review of reference lists from the relevant original and review articles.
Inclusion and exclusion criteria
Articles included in the current meta-analysis meet the following criteria: (1) the study design was the cohort study with concurrent comparison groups; (2) first-line medications had been used for active TB treatment, and isoniazid had been used for latent TB treatment; (3) participants had normal baseline liver function before starting TB treatment; (4) HBV carriers were identified as positive hepatitis B surface antigen (HBsAg), and DILI was defined as an increase in serum alanine aminotransferase (ALT) ⩾2 times the upper limit of normal (ULN; normal level ⩽40 IU/l).
Inversely, the exclusion criteria were: (1) review article, abstract, comments, letters, article with no quantitative information or details; (2) without the definition of DILI; (3) antiretroviral or hepatoprotective therapy combined with TB treatment; (4) more than 15% participants lost to follow-up.
Two investigators (JZ and MHG) independently reviewed each abstract and selected articles for the full review. The same inclusion and exclusion criteria were applied.
Data extraction and quality assessment
Data were collected independently by two investigators (JZ and MHG), and the discrepancies were resolved by consulting with a third independent investigator (HWP). The extracted data contained both qualitative and quantitative information. In particular, qualitative information included the last name of the first author, year of publication, type of study design, the region where the study was conducted, anti-TB regimens and primary definition of DILI. Quantitative data included the number of patients with liver cirrhosis and DILI in the HBV co-infection group and non-co-infection group.
Quality assessment of each included study was based on the Newcastle-Ottawa Scale, a nine-star scoring system [Reference Stang15]. For cohort studies, representation of the exposed group, the selection method of non-exposed, ascertainment of exposure, lack of outcome at baseline, comparability of groups, assessment method, enough long follow-up period and adequacy of follow-up visits were assessed. We define ⩾7 points as good quality, 6 points as fair quality and ⩽5 points as poor quality [Reference Bae16]. The two investigators independently scored the methods sections and settled the differences by consensus.
Statistical analysis
The number of eligible patients who were co-infected or not co-infected with HBV was abstracted or derived using data reported in the studies. The effect size was estimated by risk ratios (RRs) with its 95% confidence intervals (CIs). Studies were weighted by the Mantel–Haenszel method. We examined possible heterogeneity in results across studies using the I 2 statistic and χ 2 test. I 2 ⩾ 50% or P < 0.10 according to the Q statistic, which was considered to be of substantial heterogeneity, and a random-effect model was used to calculate the pooled effect size. Otherwise, a fixed-effect model was used. When there was significant heterogeneity, subgroup analyses, and meta-regression analyses based on paper language, DILI definition, study design, sample size and study participants, and TB category were conducted. Subgroup analyses could also be used to investigate clinical variability.
Furthermore, a sensitivity analysis was performed by removing each study individually to evaluate the stability and reliability of the results of the primary meta-analysis. As there were at least 10 studies included in the meta-analysis, publication bias was detected by the funnel plot and Egger's linear regression test. All statistical analyses were conducted using R software (Version 3.5.3), and P < 0.05 was considered statistically significant, except where noted.
Results
Literature search
Figure 1 shows the study selection process. We identified 1322 publications through the search of different databases. Eighteen additional articles were identified through a manual search of reference lists from the relevant original and review articles. Finally, we screened 121 full-text articles and selected 16 articles for inclusion in the meta-analysis, including 10 English-language papers and six Chinese-language papers [Reference Isa11, Reference Kim12, Reference Patel and Voigt17–Reference Chen30].
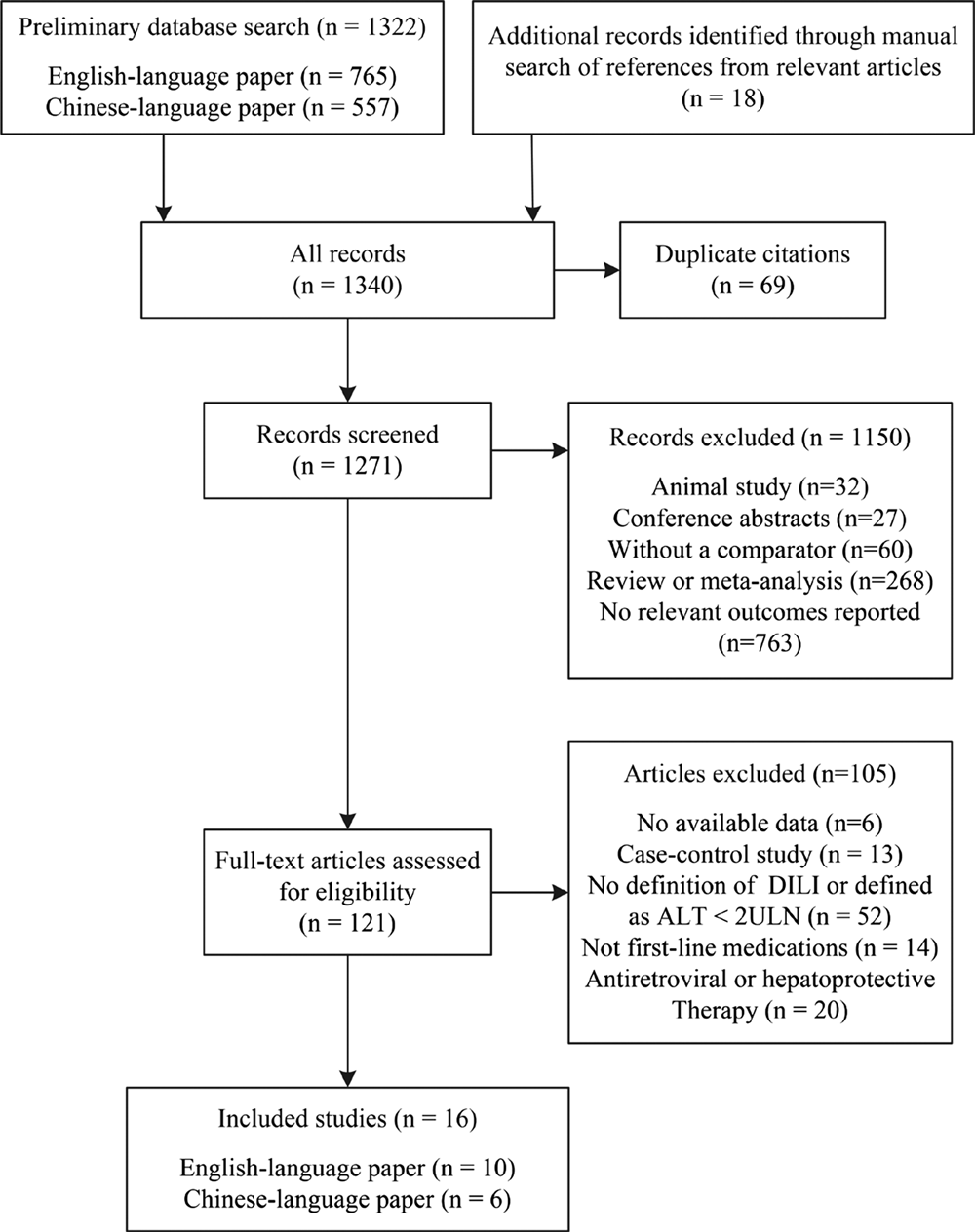
Fig. 1. Flow diagram of the study selection process.
Study characteristics
The baseline characteristics of the included studies are listed in Table 1. Of the 3960 patients who underwent TB treatment, 936 individuals with HBV infection and 3024 controls without HBV infection were enrolled in this analysis. Anti-TB regimens were based on isoniazid, rifampicin, pyrazinamide and ethambutol, except for two studies focused on isoniazid [Reference Patel and Voigt17, Reference Fernandez-Villar18]. A small proportion of participants were lost to follow-up in four studies [Reference Patel and Voigt17, Reference Fernandez-Villar18, Reference Xu24, Reference Sun28]. Eight of the 16 studies were prospective cohort studies, and the other eight studies were retrospective cohort studies. Eight of the studies focused on patients newly diagnosed with TB, and the other eight studies were not. Six studies adopted the stricter definition of DILI as serum ALT more than five times the ULN, while the other 10 studies used a looser definition of DILI (ALT ⩾ 2 or 3 ULN).
Table 1. Characteristics and quality scores of the included studies in the order of publication year
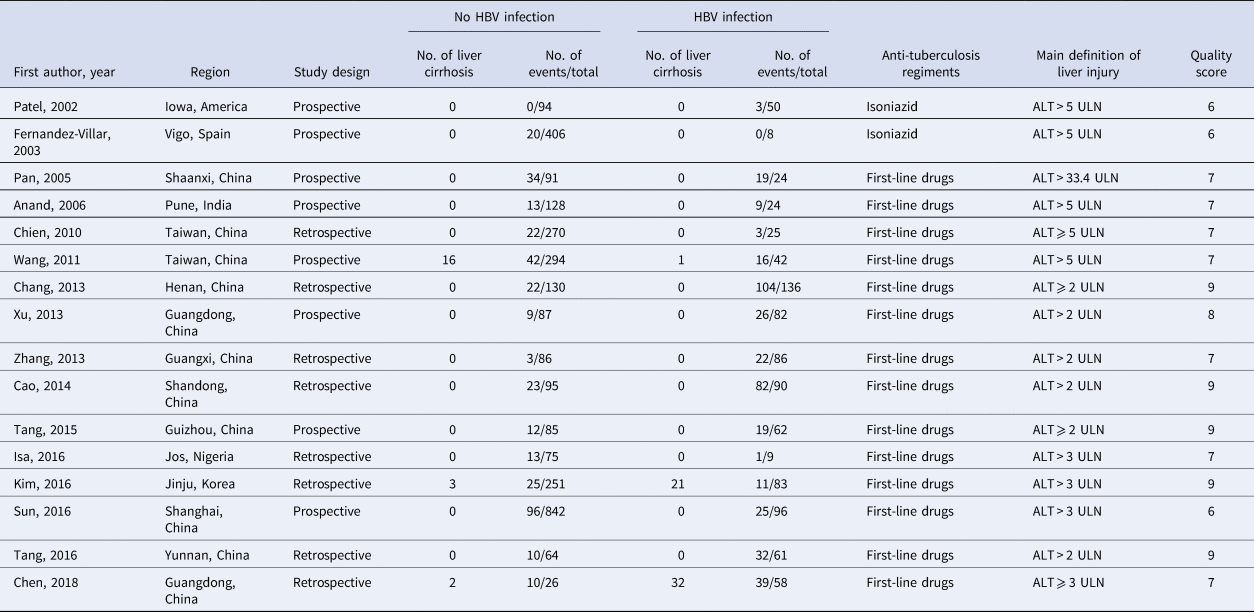
HBsAg, hepatitis B surface antigen; ULN, the upper limit of normal.
Quality assessment
The quality assessment of the eligible studies is summarised in Table 1. Five articles scored nine points; one article scored eight points; seven articles scored seven points; three articles scored six points. Common potential sources of bias included lacking information on confounding factors controlling and characteristics comparability of participants who lost to follow-up between the two study groups.
Meta-analysis
The random-effect model was selected for the significant heterogeneity (I 2 = 50%, P = 0.01) of 16 studies. Overall, TB patients with HBV infection were more likely to develop DILI (RR 2.66; 95% CI 2.13–3.32; Fig. 2) than those without HBV infection. Further analyses were performed on HBV infection patients with positive and negative hepatitis B e antigen (HBeAg) individually. Five studies provided data on positive HBeAg patients. The random-effect model (I 2 = 65%; P = 0.02) showed the pooled RR of positive HBeAg developed to DILI was 3.42 (95% CI 1.95–5.98; Fig. 3). The same five studies provided the data of negative HBeAg patients; however, an RR estimate was not calculable in one study as no patient developed DILI in either group. Thus, four studies were pooled to give an RR of 2.30 (95% CI 1.66–3.18; Fig. 3) by the random-effect model (I 2 = 23%; P = 0.28).
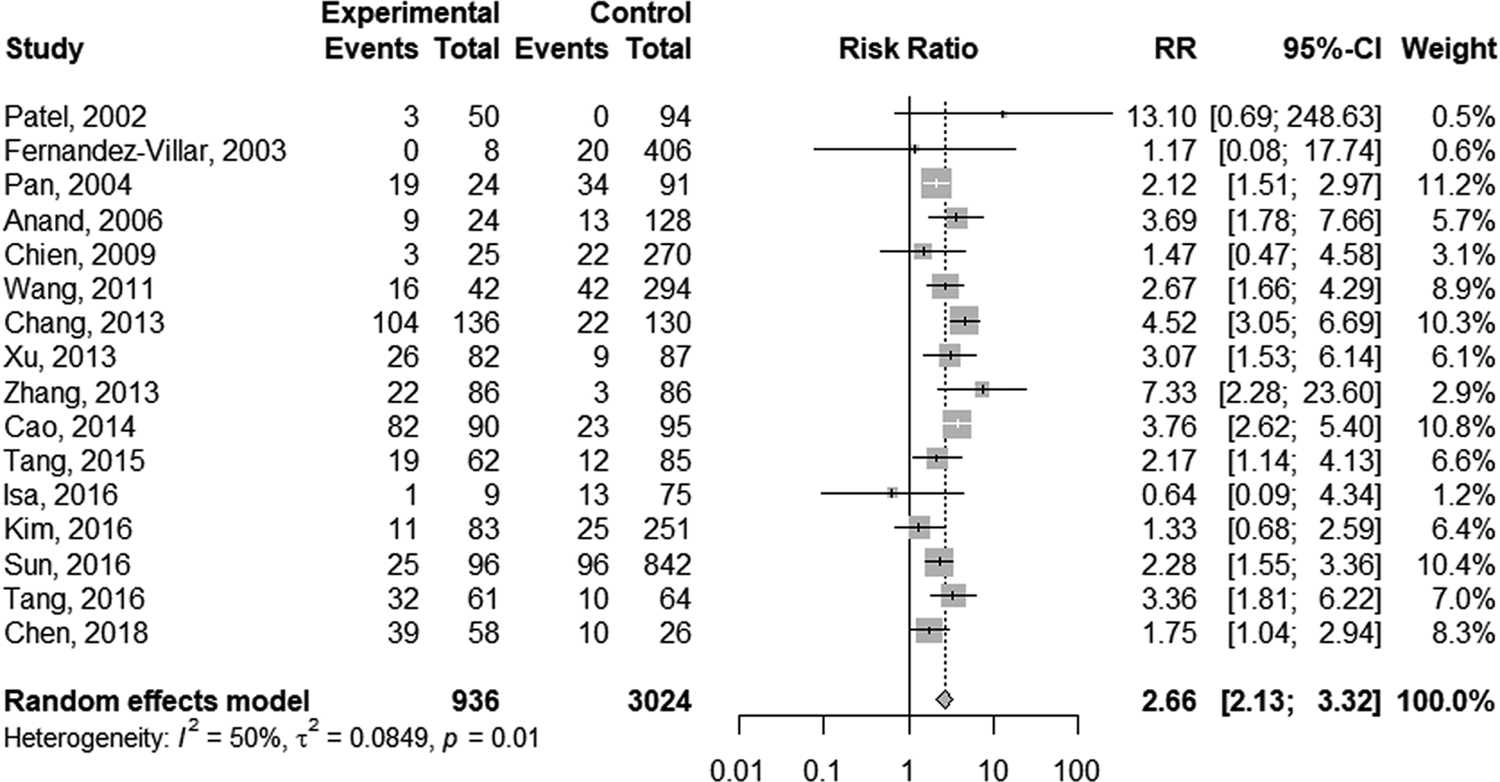
Fig. 2. Forest plot of the association between HBV infection and the risk of anti-TB DILI.
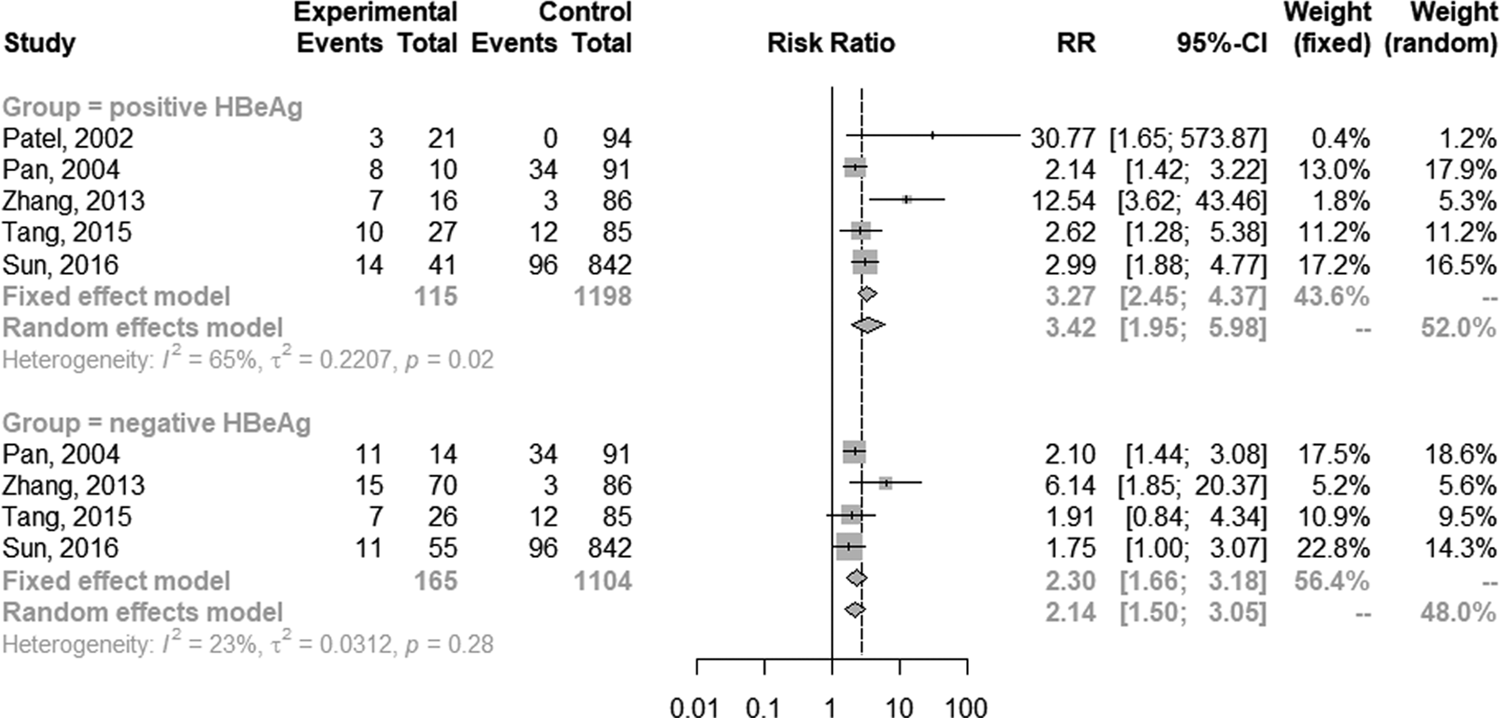
Fig. 3. Forest plot of the association between HBV infection with different HBeAg status and the risk of anti-TB DILI.
Subgroup analysis and meta-regression analysis
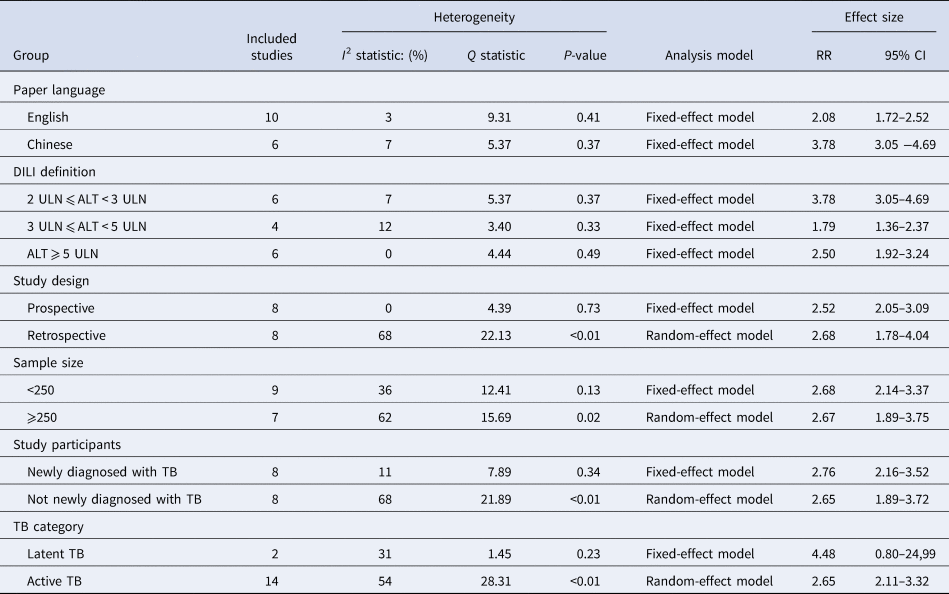
Table 2 displays the main results of subgroup analyses. The pooled RR was 2.08 (95% CI 1.72–2.52) and 3.78 (95% CI 3.05–4.69) in the studies with English-language and Chinese-language paper separately. HBV infection showed a higher risk of anti-TB DILI in the group with a loose definition of DILI (RR 3.78; 95% CI 3.05–4.69) than in the other two groups with a strict definition of DILI (RR 1.79; 95% CI 1.36–2.37; RR 2.50; 95% CI 1.92–3.24). No significant heterogeneity was found in prospective cohort studies (I 2 = 0%, P = 0.73). The fixed-effect model showed the pooled RR was 2.52 (95% CI 2.05–3.09), while the pooled RR was 2.68 (95% CI 1.78–4.04) by using the random-effect model in retrospective cohort studies. The similar effect size was detected in the patients whether they were newly diagnosed with TB (RR 2.76; 95% CI 2.16–3.52) or not (RR 2.65; 95% CI 1.89–3.72), as well as in the studies with different sample sizes (RR 2.68; 95% CI 2.14–3.37; RR 2.67; 95% CI 1.89–3.75). Besides, the fixed-effect model revealed that co-infection with HBV was not associated with a higher rate of anti-TB DILI in latent TB patients (RR 4.48; 95% CI 0.80–24.99).
Table 2. Main results of subgroup analysis and meta-regression analysis
RR, risk ratio; CI, confidence interval; ULN, the upper limit of normal; TB, tuberculosis.
Given the substantial heterogeneity among the included studies, subgroup analysis and meta-regression analysis were used to explore the explanations of heterogeneity. As studies were stratified by paper language and definition of DILI, decreased I 2 values were shown in all subgroups. Combined with meta-regression analysis results, the heterogeneity of the included studies may mainly relate to paper language and DILI definition (P < 0.001).
Duration of recovery from DILI
Four studies [Reference Kim12, Reference Chien21, Reference Tang27, Reference Tang29] showed a longer duration of recovery from DILI in HBV co-infected patients compared to non-co-infected patients (55.5 ± 62.9 vs. 15.4 ± 10.8 days [Reference Chien21]; 28.1 ± 6.4 vs. 12.9 ± 5.3 days [Reference Tang27]; 23.6 ± 4.9 vs. 14.5 ± 2.9 days [Reference Tang29]). One study presented the Kaplan–Meier curve for recovery from DILI in the two groups without reporting the recovery time. A meta-analysis on three related studies was performed to explore the pooled effect. The result showed the standardised mean difference in DILI recovery duration of patients with and without HBV co-infection was 2.26 (95% CI 1.87–2.66; Fig. 4).
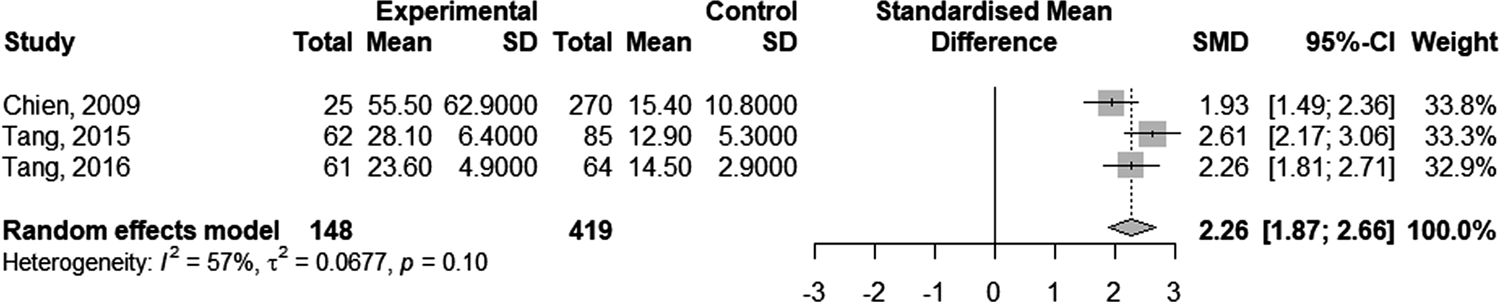
Fig. 4. Forest plot of the duration of recovery from anti-TB DILI in HBV carriers compared to non-carriers.
Sensitivity analysis and publication bias test
Figure 5 displays the sensitivity analysis results. Pooled RRs were calculated after the exclusion of one study at a time. These analyses did not reveal any notable changes in the estimates, with RRs for HBV infection and anti-TB DILI between 2.50 and 2.79. Besides, when the fair-quality studies were removed, there was no significant change. As Figure 6 showed, the funnel plot did not suggest any publication bias with the P-value for the Egger's test being 0.707.
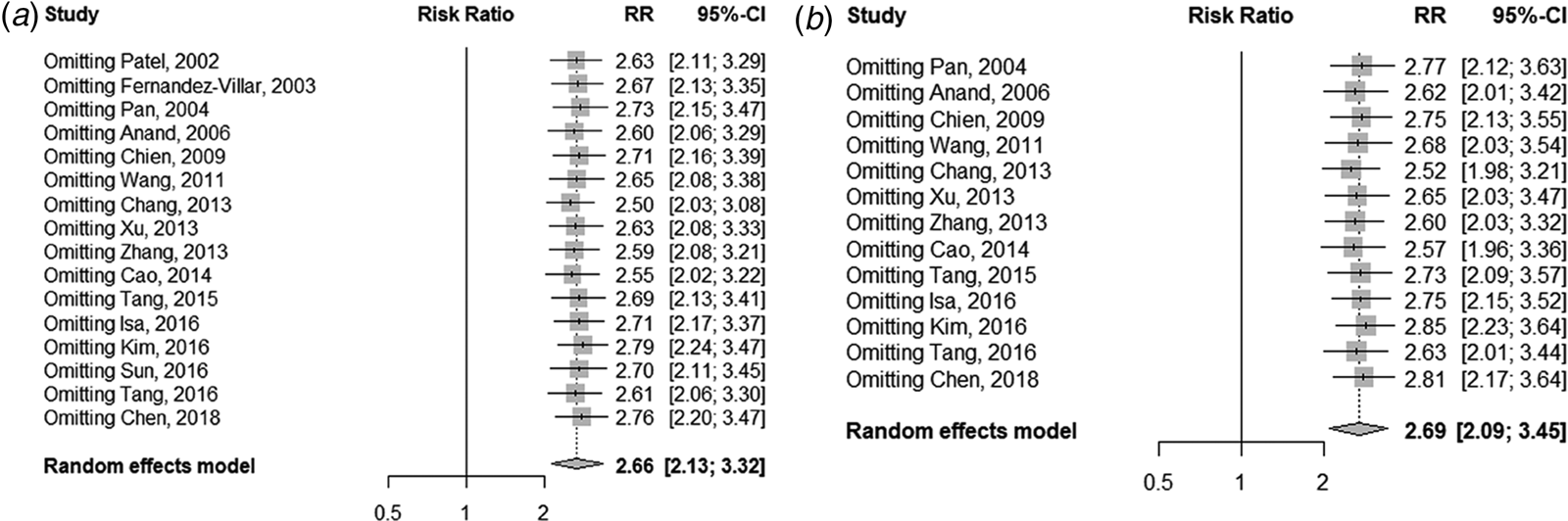
Fig. 5. Sensitivity analysis of the association between HBV infection and the risk of anti-TB DILI. (a) For all including studies; (b) excluding the fair-quality studies.
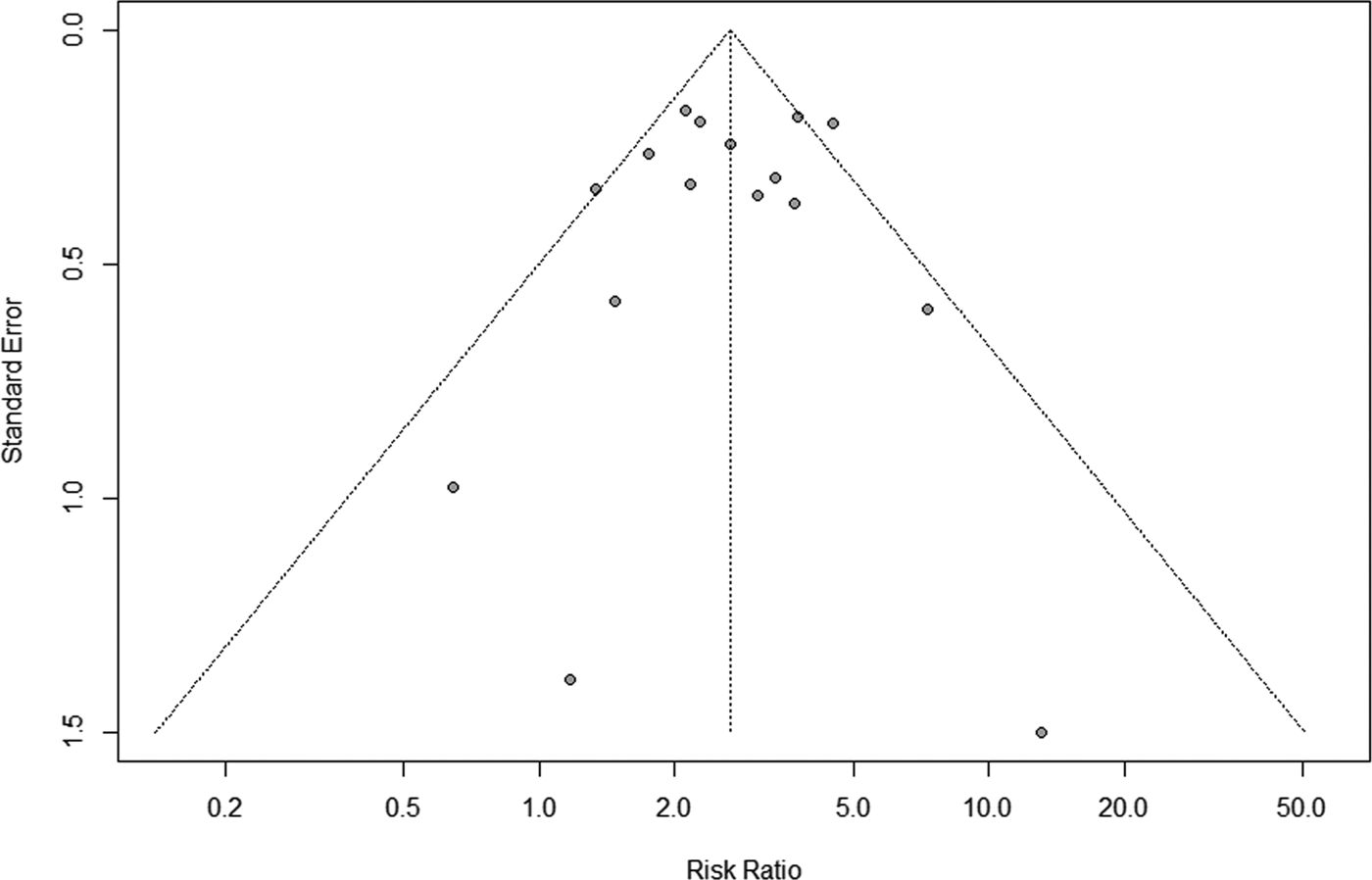
Fig. 6. Funnel plot for the assessment of publication bias.
Discussion
HBV infection is a recognised risk factor for liver injury [Reference Yuan31]. The relationship between HBV infection and the risk of anti-TB DILI remains to be explored. On analysing the pooled effect size from 16 studies, the risk of DILI appeared to be higher in TB patients co-infected with HBV than those without HBV infection. Meanwhile, four studies reported that the duration of recovery from DILI was longer in HBV co-infected patients, indicating that HBV carriers might develop DILI more severely. Furthermore, HBV infection patients with positive HBeAg were more likely to develop DILI compared to those with negative HBeAg. HBeAg is closely related to HBV replication activity so that the status of HBeAg may aggravate the interference effect of viral infection during TB therapy. The association between viral load and the risk of anti-TB DILI was not performed for the insufficient data in the included studies. Zhu et al. suggested that high levels of HBV-DNA might be a risk factor for anti-TB DILI, although the odds ratio value was 2.066, which did not achieve statistical significance [Reference Zhu32].
In the subgroup analyses, HBV infection showed a higher risk of anti-TB DILI in the group with a loose definition of DILI than in the other two groups with a strict definition of DILI. The diagnosis of DILI remains a challenge, and no recognised tests were available for physicians to establish the diagnosis [Reference Watkins33]. Hence, in our study, ALT elevation in serum was used to determine whether patients will develop serum bilirubin elevation, indicating systemic DILI. When DILI was defined as asymptomatic ALT ⩾5 ULN, the pooled RR was 2.50 with six studies. Additionally, the risk of DILI was similar in groups with different sample sizes, and the pooled effect was independent of study participants whether they were newly diagnosed with TB. There was no heterogeneity detected in the eight prospective cohort studies, which may result from closer liver function monitoring. As for the retrospective cohort studies included in our meta-analysis, all medical records were available and reviewed in detail. Furthermore, co-infection with HBV was not associated with a higher rate of anti-TB DILI in latent TB patients.
Significant heterogeneity of the included studies can be noticed, it may be explained by paper language and definition of DILI. In view of studies with English-language paper, all used a stricter DILI diagnostic criterion. Therefore, the heterogeneity may mainly relate to the DILI definition. Besides, the sensitivity analysis suggested that the pooled effect with 16 studies was robust, and no significant publication bias was detected by the funnel plot (P > 0.05).
Thus far, the shared pathogenetic mechanisms of HBV infection and anti-TB regimens inducing liver injury remain unclear. Cao et al. insisted that anti-TB drugs could generate various metabolites, which changed with the liver state of the body [Reference Cao34]. T-cell-dependent immune responses would be triggered by drugs or their metabolites, interacting with immune receptors [Reference White35]. The presence of viral infection-induced hepatocyte stress might contribute to the immune responses by raising the levels of cytokines and cell-surface markers and the expression of costimulatory molecules, which can increase the frequency of drug hypersensitivity reactions [Reference Pirmohamed36]. When HBV carriers are combined with positive HBeAg or high levels of HBV-DNA, proinflammatory cytokines and chemokines could be provoked to overexpress by actively replicating virus, which might aggravate the risk of DILI [Reference Schenker, Martin and Hoyumpa37]. Besides, HBV infection would enhance lipid peroxidation and induce reactive oxygen species accumulation due to anti-TB drugs. Further, oxidative stress destroys the normal infrastructure and activities of cells and promotes apoptosis [Reference Ha38, Reference Yew, Chang and Chan39].
Compared to the previous study [Reference Wang13], the present meta-analysis showed a similar finding that HBV co-infection might increase the risk of DILI in the first-line medication therapy for TB. Differently, our analysis only included cohort studies for the higher causal validation power and lower information bias. We used a stricter definition of DILI and provided a richer set of findings, which may facilitate providing references for clinical decision-making. Moreover, there is recent evidence that prophylactic antiviral therapy for HBV could reduce the incidence of DILI in patients with TB-HBV co-infection [Reference Lian40, Reference Lui41]. A sequential rather than an incremental approach should be administered to reduce the probability of hepatotoxicity during TB therapy [Reference Soni42].
Several factors limited the present study. First, the studies included in our analysis were all observational cohort designs without selecting study subjects randomly so that it is difficult to avoid potential selection bias and confounding factors. Second, as a majority of studies lacking data for HBeAg status in HBV carriers to develop DILI, the finding needs to be interpreted with caution. Third, although we used a stricter definition of DILI than the previous meta-analysis, it is difficult to avoid including patients with hepatic adaptation to anti-TB treatment since different studies used different definition criteria. We performed a subgroup analysis with studies using a strict vs. loose definition of DILI which may help to mitigate this defect to some extent.
In conclusion, our meta-analysis with a large sample size showed that HBV infection would increase the risk of DILI during TB therapy. Based on our findings, TB and HBV co-infection patients with positive HBeAg may have a higher risk of anti-TB DILI than those with negative HBeAg, and close liver function monitoring is needed for HBV co-infection patients whether they were newly diagnosed with TB or not.
Data
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Author contributions
XEP and YLW conceived and designed the study; JZ, MHG and HWP contributed to the collection and assembly of data; JZ and XLC contributed to data analysis and interpretation; JZ and XEP contributed to manuscript writing and review. All authors read and approved the final manuscript.
Financial support
This work was supported by the National Natural Science Foundation of China (grant number: 81473047), Joint funds for the innovation of science and technology, Fujian province (grant number: 2017Y9104), the Natural Science Foundation of Fujian province (grant number: 2019J01306), Program for New Century Excellent Talents in Fujian Province University (grant number: 2018B027), and Joint funds for the innovation of science and technology, Fujian province (grant number: 2016Y9035).
Conflict of interest
None.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors, thus ethical approval and informed consent are not required.












