The following protocol paper has been written in accordance with the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines(Reference Chan, Tetzlaff and Gøtzsche1).
Background
Depression is a common mental health disorder affecting approximately 350 million people worldwide(Reference Marcus, Yasamy and van Ommeren2). It is the leading cause of disability globally, and in Australia, it is estimated that 45 % of people will experience a mental health condition in their lifetime(3). It costs an estimated $AUD 8 billion in national employer costs annually due to loss of productivity and sick leave. A recent report suggested that even sub-clinical levels of depression represent a significant burden to the economy(Reference McTernan, Dollard and LaMontagne4). The standard treatment options include antidepressant medication and talking therapies(Reference Dimidjian, Hollon and Dobson5); although these options help many people, they may be expensive, sometimes ineffective and the medications have been found to be associated with a number of side effects(Reference Cascade, Kalali and Kennedy6). New evidence-based treatment options are urgently needed to assist with this growing health crisis.
To date, evidence suggests that diet may be a good place to start. A growing body of epidemiological evidence suggests that diet plays an important role in depression(Reference Sanchez-Villegas and Martínez-González7). Studies have looked at individual nutrients such as vitamins(Reference Swardfager, Herrmann and Mazereeuw8–Reference Almeida, Ford and Flicker10) and polyphenols(Reference Bayes, Schloss and Sibbritt11), specific foods such as fruits and vegetables(Reference Saghafian, Malmir and Saneei12) and whole dietary patterns(Reference Opie, O’Neil and Itsiopoulos13) such as traditional diets and modern diets looking at the impact these have on depression. So far, these studies have shown that healthier diets, high in vegetables and low in processed foods, are linked to better depression outcomes(Reference Quirk, Williams and O’Neil14). Currently, the diet with the most evidence is the Mediterranean diet (MD) which has also been the focus of the first intervention studies in this area. The SMILES study(Reference Jacka, O’Neil and Opie15) and the HELFIMED study(Reference Parletta, Zarnowiecki and Cho16) both showed that a Mediterranean dietary pattern can help reduce depressive symptoms in adults with depression.
In these intervention trials, the average age of participants was 40 and 44 years and both studies recruited more women than men. However, three quarters of mental illnesses occur before the age of 24 years(Reference Kessler, Berglund and Demler17), and common mental health disorders, such as major depressive disorder, are commonly episodic and risk re-emergence during young adulthood. Additionally, the disability-adjusted life years and rates of mortality due to mental health are also highest among emerging adults (aged 18–29 years) compared with any other age group(Reference Whiteford, Ferrari and Degenhardt18); thus, prevention or early treatment of mental disorders should focus on this demographic.
A recent systematic literature review assessed the associations between diet quality and common mental disorders in emerging adulthood(Reference Collins, Dash and Allender19). The authors highlight that emerging adulthood presents a particularly risky period for unhealthy dietary behaviour and poor mental health due to significant transitions between home, employment and education(Reference Collins, Dash and Allender19). Additionally, emerging adulthood represents a transfer of agency where many young people become responsible for their meals and eating habits for the first time(Reference Collins, Dash and Allender19). Therefore, dietary interventions aimed during this critical period warrant attention. Research has also found that men are less likely than women to seek help for their depression(3) with only 13 % of young men aged 15–24 years seeking help for their mental health(Reference Slade, Teesson and Burgess20). Interventions targeting young adults, and young men specifically, are therefore of high importance. A healthy MD may provide a tangible goal for new mental health treatment and prevention in this demographic.
Studies consistently show differences in the food choices and behaviours of men and women(Reference Beer-Borst, Hercberg and Morabia21–Reference Davy, Benes and Driskell23). Men tend to eat fewer fruits and vegetables and consume more high sugar drinks and alcohol than women(Reference Wardle, Haase and Steptoe24). A survey of young adults found that men were significantly less likely to engage in food preparation behaviours compared with women(Reference Larson, Perry and Story25). With another study of young adults finding that men are more than twice as likely to consume takeaway food twice a week or more compared with women(Reference Smith, McNaughton and Gall26). A recent study explored the diets, nutritional knowledge and opinions of 384 young men aged 18–25 years with depression(Reference Bayes, Schloss and Sibbritt27). Two-thirds of participants stated that they notice an impact of diet on their mental health, and 77 % reported that they would be likely to change their diet if it helped their depression(Reference Bayes, Schloss and Sibbritt27).
Clearly, there are several differences in the eating habits of men and women, with men generally displaying poorer food choices. When considering both the poorer diet quality and reduced help seeking behaviour of young men, coupled with the need to target early depression treatments on youth, more studies in this demographic would be beneficial. Thus, young men with depression and poor dietary habits are an ideal group to test the effect of a healthy MD.
Methods
Research aims and objectives
The primary outcome of this study is to evaluate the effect of a MD on young men with major depressive disorder. The study aims to determine if the effect of the MD differs significantly from that of the control group by comparing the twenty-one-item Beck’s Depression Inventory (BDI-II)(Reference Dozois, Dobson and Ahnberg28) results between the intervention and control groups. Secondary aims are to see if there is a difference in outcome measures between participants who display a high v. low diet compliance. This will be achieved by comparing the depression scores between participants who have a high MD compliance score and those who have a low MD compliance score on an adapted validated fifteen-item MD adherence questionnaire. Last, the study aims to understand the attitudes, perceived benefits and challenges of continuing to follow a MD by young men with depression by uncovering the perceived challenges and benefits to following a MD in the End of Project Evaluation Survey.
Study design
Data were collected from twenty young men aged 18–25 years diagnosed with depression which helped inform the design of this study. The men completed a short qualitative questionnaire consisting of seven open-ended questions which invited them to share their opinions on the methodology of this trial. The following research protocol incorporates that data and informed several aspects of the trial including dietary support documents, number and duration of follow-up visits and diet recording and reporting methods.
The AMMEND study is a 12-week randomised controlled trial testing the effect of a MD on the symptoms of depression in young men. Participants will be randomly allocated to either the MD group or the control group which consist of the social support procedure, befriending. Participants will attend three 45-min online appointments at baseline, week 6 and week 12. Data collection will include case report forms (CRF) and several questionnaires.
Study population and sampling
The study population consists of young men aged 18–25 years who have been diagnosed with major depressive disorder by a general medical practitioner. The study sample will be selected using random sampling.
Power analysis
The sample size calculation was based on being able to detect a minimum clinically important change of >5 points in BDI(Reference Masson and Tejani29) while comparing diet and placebo group, with α = 0·05 and 80 % power. In previous studies(Reference Tayama, Ogawa and Nakaya30,Reference Sepehrmanesh, Kolahdooz and Abedi31) , the response within each subject group was normally distributed with an approximate sd of 7·5. Based on this, thirty-six participants are needed per group. Allowing for a 15 % dropout rate, we are aiming to recruit eighty-six participants.
Recruitment
Recruitment will be online and consists of the following strategies. Social media advertisements via Facebook and Twitter which target the following demographic: individuals who are male, aged 18–25 years, live in Australia and have ‘liked’ or ‘followed’ pages relating to depression such as the Back dog institute, beyond blue and Sane. An email campaign which includes a link to the online website (https://mendds.wixsite.com/ammend) and has all the relevant information about the trial will be sent to Australian-based medical doctors, psychologists, natural health practitioners and health clinics, with a view to these practitioners notifying patients who meet the study criteria, about the study and providing the patients with the website link. Practitioners contact details will be collected from a thorough Internet search. All participants who are referred by allied health practitioners will be screened to check eligibility. A referral letter stating that the participant has been diagnosed with major depressive disorder will also be requested.
Inclusion and exclusion criteria
The study population will consist of young men aged 18–25 years who have been diagnosed with major depressive disorder by their medical doctor. They must also score 20 or above on the twenty-one-item BDI-II indicating moderate to severe depression and have scored <40 on the Commonwealth Scientific and Industrial Research Organisation Diet Survey(Reference Hendrie, Baird and Golley32) indicating a poor baseline diet. Participants will be excluded for the following reasons: (1) If they cannot speak or understand English or if they are not capable of understanding or consenting to what is involved with this trial. (2) If they have also been diagnosed with any of the following mental health disorders: bipolar disorder, post-traumatic stress, personality disorders, eating disorders, psychotic disorders such as schizophrenia or a substance abuse disorder such as alcoholism. (3) If they suffer from any gastrointestinal disorders such as Crohn’s disease, ulcerative colitis or irritable bowel syndrome. (4) If they have any food allergies, intolerances or eversions (avoiding foods based on religious or ethical grounds) which would prevent them from following the diet. (5) If they are unavailable to attend the scheduled follow-up appointments. (6) If they are unwilling to change their diet if allocated to the MD group. (7) If they score below 20 on the BDI-II or if the BDI-II indicates suicidal thoughts or ideations.
Screening procedure
A phone screening tool specifically developed for this study will be used to cover all aspects of the inclusion and exclusion criteria. This includes the Beck’s Depression Inventory to assess depressive symptoms and the Commonwealth Scientific and Industrial Research Organisation diet questionnaire to assess current diet quality. If the participant is deemed eligible, they will be sent the participant information sheet and scheduled a baseline appointment. A computer-generated random number sequence will be used for randomising participants. The Chief Investigator will generate the sequence, and the Principle Investigator will conceal the sequence in numbered sealed opaque envelopes. Participants will be allocated a sequential number upon screening. The sealed envelope corresponding to the allocated participant number will be opened at the baseline appointment by the researcher, and the participant will be informed which group they have been randomised into. As this is an open-label study, blinding is not required. A Consolidated Standards of Reporting Trials (CONSORT) flow chart outlining the study schedule is displayed in Fig. 1.
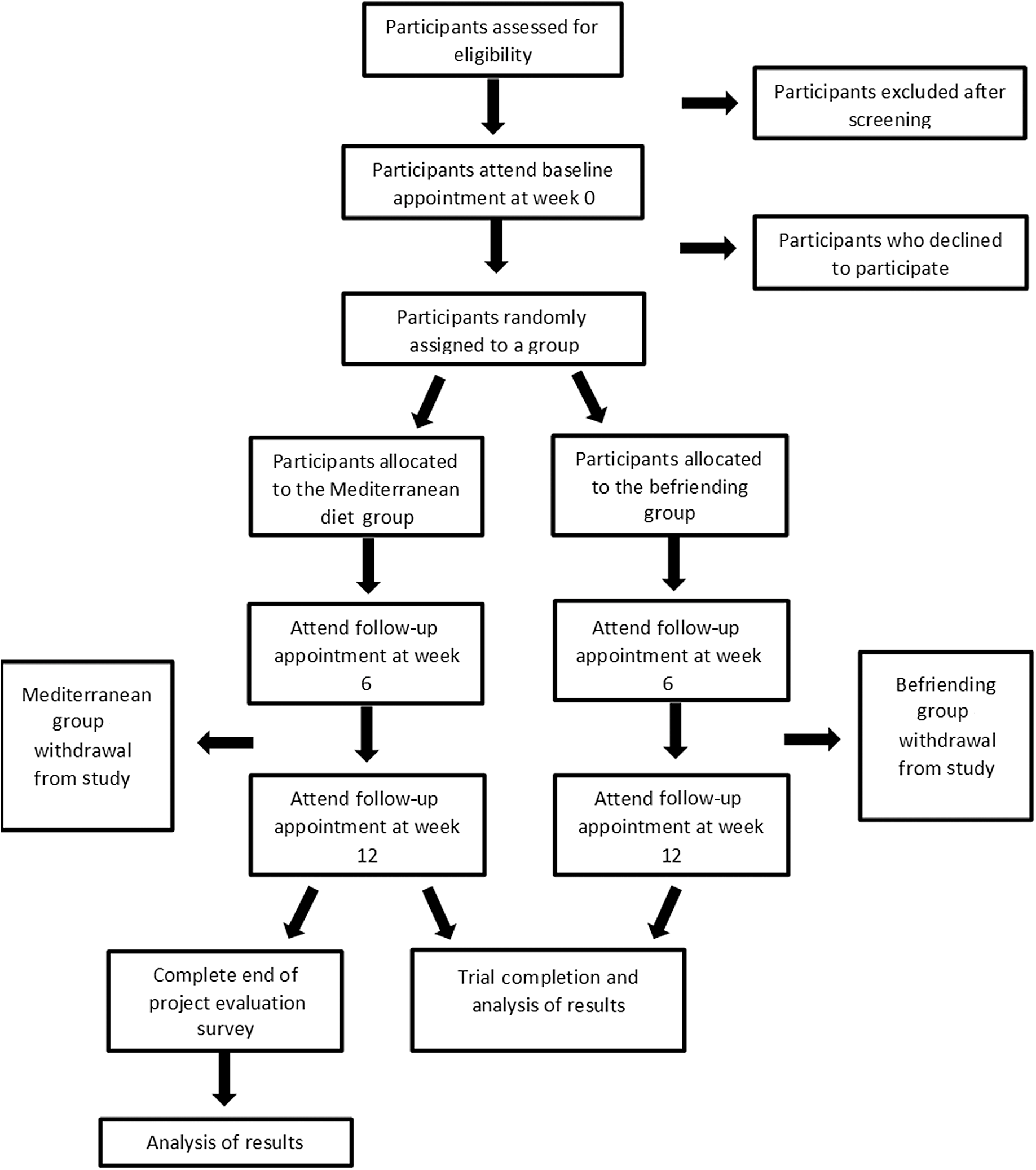
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flow chart.
Diet intervention group
Participants allocated to the MD intervention group will receive nutrition consultations by a qualified nutritionist explaining the components of the diet. The MD used in this study is based on the dietary guidelines of Greece and Spain. The diet is rich in vegetables, legumes and wholegrains, oily fish, olive oil and raw unsalted nuts. The primary focus is on increasing diet quality with fresh wholefoods while reducing intake of energy-dense, nutrient-poor ‘fast’ foods. They will be provided with a booklet containing sample meal plans, recipes, dining-out options, simple diet ‘swaps’, eating-on-a-budget tips, compliance checklists and online diet history survey link.
Participants will also receive a food hamper at the commencement of the trial. The follow-up appointment at 6 weeks will involve a 45-min consultation where participants will complete another CRF including BDI-II and WHO Quality-of-Life (QOL-BREF) form. Participants will be asked about their experiences so far and will receive additional nutritional counselling. Participants will attend a final appointment at the conclusion of the study where the final BDI-II will be taken. Diet history will be collected daily from participants via a widget on their mobile device. Participants will be invited to take part in an End of Project Evaluation Survey at week 12, collecting information about their experiences following a MD and their depressive symptoms.
Control group
Participants allocated to the control group will receive 45-min befriending support sessions. These will be scheduled for the baseline appointment, 6 weeks and 12 weeks in order to mimic the visit schedule and duration of the diet intervention group. Befriending consists of the researcher talking to the participant about neutral topics of interest to the participant such as sports, movies and hobbies. The objective is to keep the participant engaged and interested. The befriending protocol has been selected because it controls for several factors including client expectations, the therapeutic relationship and time spent with the nutritionist. Befriending is often used as a controlled condition for clinical trials of psychotherapy(Reference Bendall, Jackson and Killackey33) and was used recently in the SMILES trial(Reference Jacka, O’Neil and Opie15). Befriending has shown to be an effective, credible and acceptable validated control therapy for psychological studies and appropriate to participants suffering from mental illness(Reference Bendall, Jackson and Killackey33). Participants will also complete the CRF, BDI-II and WHO QOL forms at their baseline appointment, 6-week follow-up and final appointment at week 12. Participants in the control group will also receive a $50 Hoyts gift card to thank them for their participation and to act as incentive to return for their final appointment for data collection.
Data collection methods and instruments
Data will be collected from all participants via CRF and will include the twenty-one-item BDI-II depression scale and the WHO Quality-of-Life (QOL-BREF) form. Adherence to the MD will be measured via an adapted validated fifteen-item MD Adherence Scale. All participants, both the diet and control groups, will be required to report everything they eat and drink online daily.
Case report form
The CRF includes demographic questions, a section to report all current medications, both prescription and over the counter, nutritional supplements and herbs as well as complementary medicine use. Participants are asked not to change any aspect of their usual routine for the duration of the trial, particularly exercise and use of vitamins. These activities will be recorded at each appointment. Energy, stress levels and other lifestyle factors will also be recorded. An adverse event log is also included.
Beck’s Depression Inventory (twenty-one-item)
The twenty-one-item BDI-II is one of the most popular self-assessment tools for depression and has been used in over 7000 studies worldwide(Reference Wang and Gorenstein34). The twenty-one items in the BDI-II reflect symptoms and attitudes observed in the criteria for depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders. It demonstrates high reliability and good correlation with measures of depression and anxiety(Reference Wang and Gorenstein35). Each of the twenty-one items has four possible answers rating from zero to three. These reflect their intensity and create a score which ranges from 0 to 63(Reference Wang and Gorenstein34). A score of 20 and above indicates moderate depression and forms part of the inclusion criteria for the trial. The BDI-II has also demonstrated a high level of validity, sensitivity and specificity for detecting depression in both the general population, and psychiatric and medical settings(Reference Lasa, Ayuso-Mateos and Vazquez-Barquero36). A systematic literature review of the psychometric properties of the BDI-II found that it reports an average alpha reliability coefficient of 0·9, ranging from 0·83 to 0·96(Reference Wang and Gorenstein34). It also reports the retest reliability (Pearson’s r) as relatively stable with good to excellent coefficients (range 0·73–0·96)(Reference Wang and Gorenstein34).
The Commonwealth Scientific and Industrial Research Organisation Healthy Diet Score
The Commonwealth Scientific and Industrial Research Organisation Healthy Diet Score Survey asks questions about the quantity, quality and variety of foods consumed(Reference Hendrie, Baird and Golley32). Individuals receive a personalised Diet Score out of 100 which reflects their overall compliance with the Australian Dietary Guidelines. Over 145 000 Australians have completed the survey since it was launched in May 2015(Reference Hendrie, Baird and Golley32). The average Diet Score was 58·8 out of a possible 100. A score of 75 has been proposed as a benchmark of a ‘good’ score(Reference Hendrie, Baird and Golley32). A score of 40 or below would thus indicate a ‘poor’ score and forms part of the inclusion criteria for the AMMEND trial.
WHO Quality-of-Life form
The WHO QOL-BREF is a twenty-six-item version of the WHO QOL-100 assessment. The WHO QOL form has demonstrated to be a thorough, cross-culturally valid assessment of QOL, as reflected by its four domains: physical, psychological, social and environment(Reference Skevington, Lotfy and O’Connell37). WHO QOL-BREF has been used previously in participants with major depressive disorder and was sensitive to improvement after treatments(Reference Berlim, Pavanello and Caldieraro38). It has shown to be a psychometrically reliable instrument that it is suitable for evaluating quality of life.
The Mediterranean Adherence Score(Reference Papadaki, Johnson and Toumpakari39)
The Mediterranean Adherence Score (MEDAS) is a fourteen-item questionnaire which asks about the frequency of consumption or amount consumed of twelve main components of the MD and two food habits related to the MD. Each of the fourteen items is scored 1 or 0, depending on whether participants adhere to each MD component or not(Reference Papadaki, Johnson and Toumpakari39). Participants total score will be categorised into two groups: <7 points (low adherence) and ≥7 points (high/medium adherence). The English version of the MEDAS has displayed acceptable accuracy and reliability for assessing MD adherence(Reference Papadaki, Johnson and Toumpakari39) and has been used in depressed populations previously(Reference García-Toro, Vicens-Pons and Gili40). An additional question about whole-grain servings has also been included in our adapted version of this questionnaire: How many serves of whole grains (wholemeal bread, brown rice, oats, etc.) do you consume per day? A point is awarded for ≥3 serves. Participants daily diet entries will be scored against the MEDAS and given an average score at the conclusion of the study.
Diet guidelines
The term MD has often been misused. There is no evidence that one ‘Mediterranean Diet’ is followed by everyone living in the countries around the Mediterranean Sea. However, the foods that are common among the various countries of the Mediterranean regions are somewhat similar. The healthy MD is a moderate diet characterised by some typical Mediterranean food groups: cereals, legumes, fish, virgin olive oil, fresh fruit, nuts, vegetables, wild plants and wine as an optional alcoholic beverage. The MD used for the AMMEND study is based on the dietary guidelines of Greece and Spain. It is abundant in plant foods including vegetables, fruits, cereals, legumes, nuts and seeds. Olive oil is the principle source of fat, dairy products (principally cheese and yogurt) and fish and poultry are consumed in low to moderate amounts, zero to four eggs consumed weekly and low red meat consumption.
Participants will document all meals and snacks consumed during their time in the study via a widget on their mobile device. A score is calculated based on the MEDAS detailed earlier.
End of Project Evaluation Survey
The End of Project Evaluation Survey is a twenty-item questionnaire consisting of sixteen multiple choice questions and four open-ended questions asking participants in the diet group to reflect on their experiences following the MD. The Survey asks participants to reflect on several different topics including their weekly food budget, time commitment, motivation, enjoyment level, challenges and perceived impact on their depressive symptoms.
Data analysis methods
Statistical analysis will be performed using STATA. Descriptive statistics will be reported using means and standard deviations providing baseline demographics and measurements. The two groups will be compared across baseline measurements, using χ 2 or t tests where appropriate, to ensure that there are no differences between randomised groups. If differences between the two groups are identified, those variables will be included in linear regression modelling of the outcome variable. Possible confounders such as changes to exercise, stress, sleep, recreational drug use, over-the-counter medications and supplement use will be considered in the statistical analysis.
The main outcome will be the BDI-II score which will be analysed as a continuous variable and reported as a mean. A one-tailed analysis will be used to detect differences in BDI-II scores between the intervention and control groups. Intention-to-treat analysis will be used exploring dose–response effects associated with the MD adherence. Changes between BDI-II score at baseline and the conclusion of the study will be analysed using paired t tests. Differences in BDI-II scores between both groups will be assessed using a linear regression model. The BDI-II can also be split into two subgroups: Cognitive–affective items (Cognitive–Affective subscale) and somatic and performance complaints (Somatic and Performance subscale). Analysis of the effect of the MD on the two subscales will also be performed.
To investigate whether dietary change is associated with positive outcomes in depression, changes in diet scores will be entered into regression analyses as appropriate with changes in BDI-II scores as dependent variables. Sub-group analyses by depression severity score will be conducted to determine if the severity of depression affects the results.
Secondary aims are to see if there is a difference in outcome measures between participants who display high v. low compliance to the MD. The final BDI-II score of participants who display low compliance (<7 on the MEDAS) will be compared with those who display moderate to high compliance (≥7 on the MEDAS) with linear regression modelling.
Data management
All data will be collected and stored according to Good Clinical Practice Guidelines. All hard copy data such as consent forms, CRF, dietary screening forms and BDI-II scores will be stored in a locked filing cabinet which can only be accessed by the lead researchers. Electronic data will be stored securely in REDcap and a Research Data Management Plan created via Stash. Data will be stored for 5 years, and all information will be treated confidentially. Data will only be used for the purpose of this research project.
Access to data
Data can only be accessed by members of the research team. If requested, participants can access their individual results at the completion of the trial by contacting the lead researcher.
Data monitoring and auditing
Data monitoring and auditing of the trial will be conducted by the senior researchers and supervisory team D. S. and J. S.
Ethical considerations
Due to this trial involving participants with depression, extra caution will need to be taken. Participants will have been previously diagnosed with depression by their general practitioner and will be under their primary care. Participants will continue with their prescribed treatment protocol whether that be medications and/or counselling during the course of the trial. If there are any changes to their depression treatment during the course of the trial, these will be noted on the CRF and reported as confounders. This is a pragmatic trial and represents real-life events. Participants will be specifically asked about any changes to medications, either type or frequency, psychology appointment frequency or complementary therapies/herbal remedies at the follow-up appointments. A letter communicating the participants’ involvement in this study will be given to the participants’ primary care doctor explaining all aspects of the trial. During the study, the BDI-II will be completed at baseline, week 6 and week 12. While we do not expect that a MD or befriending will cause a significant worsening of symptoms, as both have used safely in previous depression clinical trials, we will still carefully monitor BDI-II results.
Ancillary and post-trial care
If the results indicate a worsening of symptoms during or after the study, the participants’ doctor/counsellor will be contacted. If the participants’ health is at risk, they may be withdrawn from the study. A complementary counselling session will also be organised with the study Psychologist. Participants will be aware that they can withdraw from the trial at any time and for any reason. A detailed handout of depression resources will also be given to participants at the commencement of the trial. There is also a possible risk of minor gastrointestinal symptoms as a result of dietary changes. These include bloating, altered bowel movements and abdominal pain as a result of increased fibre and introduction of new foods. If these symptoms occur, they will be ameliorated by the qualified clinical nutritionist and recorded in the Adverse Events Log. The trial may be terminated early if the results suggest that the diet or social support is causing harm. This decision will be made by senior members of the research team, D. S. and J. S.
Study integrity
This trial has been designed following Good Clinical Practise principles in line with the declaration of Helsinki, and all researchers hold current Good Clinical Practise certificates. Ethical Approval was granted by the University of Technology Sydney on 4 February 2020: University of Technology Sydney HREC reference no. ETH19-4413. The trial is registered with Australia and New Zealand Clinical Trials Registry Trial ID: ACTRN12619001545156 and has also been registered with the WHO International Clinical Trials Registry Platform’s Universal Trial Number: U1111-1242-5215.
Declaration of interests
This project is funded by Endeavour College of Natural Health. This is an independent research project. The funding body has no involvement with the design, implementation or analysis of the project. Their only role is providing the money awarded by the grant approval. There are no potential commercial interest to declare.
Dissemination policy
The results from this study will be published in a peer-reviewed academic journal. Participants in the trial will also be notified of the outcomes. A progress report will also be completed for Endeavour College of Natural Health.
Discussion
The SMILES(Reference Jacka, O’Neil and Opie15) and HELFIMED(Reference Parletta, Zarnowiecki and Cho16) trials have both shown that a MD can be effective at reducing depressive symptoms in adults with depression and are supported by a growing body of observational research. This project aims to assess the MD in a very specific population group often underrepresented in diet research. Unfortunately, young men rarely seek help for their depression, which suggests that the current treatment options are particularly unappealing for this demographic and the stigma of depression is still significant. Evidence-based treatment options for young men are urgently needed, and this research aims to answer the question of whether diet can be used effectively in this population.
Changing behaviours is challenging, and diet research requires intense commitment from participants. A previous study found the following facilitators and barriers for adopting a MD in a non-Mediterranean country(Reference Middleton, Keegan and Smith41). Participants reported that the diet was enjoyable and that they experienced pleasure and fulfilment at meal times but that stress or work pressures often got in the way and made it difficult to prepare food(Reference Middleton, Keegan and Smith41). The authors also recommend challenging assumptions about what a MD looks like and educating participants that the MD is not a ‘salad only’ diet. They also recommend education on where to find certain foods and meal planning skills(Reference Middleton, Keegan and Smith41). These factors have all been considered in the design of this study and appropriate time set aside to discuss these issues with participants.
Potential limitations of this trial include selective dropout/retention from participants not being allocated to their preferred intervention group. To control for this, participants in each intervention group will be offered the opportunity to try the alternative intervention at the conclusion of the study. For example, participants allocated to the social support group can receive nutritional counselling if they wish, once they have finished their trial period. Other behavioural exposures could also impact the trial results. These will be controlled for by asking extensive questions in the CRF about possible confounders such as exercise, stress, sleep, recreational drug use, over-the-counter medications and supplement use. These factors will then be considered in the statistical analysis. As this is a whole diet intervention, this trial cannot be double-blinded; however, the two intervention groups will be presented as equal in their possible treatment efficacy to reduce client expectancy.
Despite these limitations, this study has a number of strengths. These include that the participants depression is diagnosed by a general practitioner rather than being self-reported and the randomised nature of the trial preventing intervention selection bias and confounding. Another strength is the use of befriending for the social support group which controls for several potential confounders including the therapeutic relationship and the time and attention received by the participant. This will also be the first trial to assess a whole diet approach in young men with depression. Whole diet approaches consider food synergy and how foods work together naturally within the diet. This reflects a more natural way of eating rather than consuming isolated nutrients. An additional strength of this design is that by collecting diet history information daily, there is a reduced risk of recall or measurement error. Further, the follow-up survey included at the conclusion of this trial will help inform future research by highlighting the positive and negative aspects of following a MD by this demographic and will help researchers and clinicians implement this diet in a real-world setting.
Conclusion
This will be the first randomised clinical trial to assess the impact of a MD on the symptoms of depression in young men. This study will not only help fill a significant research gap but also contribute to the growing field of nutritional psychiatry. The results from this study may also help guide future research in this area and inform advice given by clinicians to this specific demographic.
Acknowledgements
J. B. would like to acknowledge the support of the Australian Government Research Training Program Scholarship.
The authors would like to thank Endeavour College of Natural Health and the Australian Research Centre in Complementary and Integrative Medicine (ARCCIM), University of Technology Sydney (UTS) for providing funding for this research.
J. B. conceptualised and designed the study; J. S. and D. S. assisted with the development of the study design, methodology and statistical analysis plan; J. B. obtained the funding and ethics approval with assistance from J. S. and D. S.; J. B. drafted the study protocol with edits from J. S. and D. S.; all authors contributed to the manuscript and approved the final version.
There are no conflicts of interest, and no competing financial interests exist.






