Introduction
Brazil is home to 115 species of non-human primates (Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque and Di Fiore2017; Costa-Araújo et al., Reference Costa-Araújo, Melo, Canale, Hernández-Rangel, Messias and Rossi2019; IUCN, 2019), of which 21 occur in the Atlantic Forest, Cerrado and Caatinga biomes in the north-east. These three biomes have been extensively modified by centuries of anthropogenic forest destruction for the development of agriculture, infrastructure and urban areas. Primate populations are in sharp decline, including the most charismatic, elusive and rare species. Half of the primate species in north-east Brazil are under threat, including five species categorized as Endangered and five categorized as Critically Endangered on the IUCN Red List of Threatened Species (IUCN, 2020). Although much attention has been, rightfully, devoted to the plight of the Brazilian Atlantic Forest, there has been little focus on the Cerrado and Caatinga. The Caatinga in particular has been neglected in terms of conservation action, although nearly half has already been lost (Beuchle et al., Reference Beuchle, Grecchi, Shimabukuro, Seliger, Eva, Sano and Achard2015). It is predicted that the Atlantic Forest, Cerrado and Caatinga biomes will be severely affected in the future by continuing anthropogenic impacts in these rapidly developing areas, and by climate change (Marengo et al., Reference Marengo, Torres and Alves2017). Thus it is important to determine which areas will be more severely affected and where new protected areas are required to create an effective network of protected areas that supports the future survival of primates in these biomes (Estrada et al., Reference Estrada, Garber, Mittermeier, Wich, Gouveia and Dobrovolski2018).
In 2011 the Instituto Chico Mendes de Conservação da Biodiversidade, the national institution for biodiversity conservation in Brazil, developed a conservation action plan for the primates of north-east Brazil (ICMBio-CPB, 2018). Six of the 21 primate species in the area were included in this conservation action plan: the red-handed howler monkey Alouatta belzebul, Caatinga howler monkey Alouatta ululata, blonde titi monkey Callicebus barbarabrownae, Coimbra-Filho's titi monkey Callicebus coimbrai, blonde capuchin Sapajus flavius and yellow-breasted capuchin Sapajus xanthosternos. These species occur in one or more of the three biomes occurring in north-east Brazil, the Atlantic Forest, Cerrado and Caatinga. The main threats to these primates are habitat destruction and fragmentation, hunting and the pet trade (ICMBio, 2016; ICMBio-CPB, 2018). One of the actions proposed in the conservation action plan is to determine more accurately the current distribution of all six species and to evaluate how these distributions will be affected by future human activities and climate change (ICMBio-CPB, 2018). Such data are essential for conservation planning, to ensure there is sufficient suitable habitat for the survival of these species through creating new protected areas, connecting existing ones and restoring key habitats.
Spatial analyses such as species distribution models and gap analyses are useful for assessing the impact of habitat loss and fragmentation on species (Beuchle et al., Reference Beuchle, Grecchi, Shimabukuro, Seliger, Eva, Sano and Achard2015; Titeux et al., Reference Titeux, Henle, Mihoub, Regos, Geijzendorffer and Cramer2017; Zwiener et al., Reference Zwiener, Lira-Noriega, Grady, Padial and Vitule2018). Distribution models project potentially suitable areas for a particular species based on presence location records and abiotic environmental data (Elith & Leathwick, Reference Elith and Leathwick2009). Gap analysis assesses whether species or ecosystems are represented within existing protected areas; i.e. it identifies potential conservation gaps (Rodrigues et al., Reference Rodrigues, Andelman, Bakarr, Boitani, Brooks and Cowling2004). Both techniques provide information to guide efficient management actions for the conservation of a greater number of species (Rodrigues et al., Reference Rodrigues, Andelman, Bakarr, Boitani, Brooks and Cowling2004), and, if integrated, improve the interpretation of impacts of global change scenarios on biodiversity (Titeux et al., Reference Titeux, Henle, Mihoub, Regos, Geijzendorffer and Cramer2017). The combination of such approaches is thus important for conservation planning, particularly because research and conservation efforts have traditionally focused on charismatic species and protected areas, leaving some species and their habitats at high risk of extinction (Bezanson & McNamara, Reference Bezanson and McNamara2019).
Here we combine species distribution models and gap analysis to assess the long-term suitability of habitats for the conservation of two of the six target species included in the conservation action plan: A. belzebul and S. flavius. We also include a third species, the bearded capuchin Sapajus libidinosus. This species is not included in the national action plan but is strongly affected by habitat loss (Beuchle et al., Reference Beuchle, Grecchi, Shimabukuro, Seliger, Eva, Sano and Achard2015; Rylands & Kierulff, Reference Rylands and Kierulff2015), the illegal pet trade (Nascimento et al., Reference Nascimento, Schiavetti and Montaño2013) and hunting for use in traditional medicine and in retaliation for crop use (Torres Junior et al., Reference Torres Junior, Valença-Montenegro and de Castro2016; Freire-Filho et al., Reference Freire-Filho, Pinto and Bezerra2018; Souto et al., Reference Souto, Barboza, Fernandes-Ferreira, Júnior, Monteiro, Abi-chacra and Alves2018). We chose these three species because their habitats represent the main biomes of north-east Brazil and they could thus serve as flagship species, with their protection providing wider benefits for the conservation of these habitats and other wildlife within them. We (1) estimate the current potential range of each species and project the effects of future climate change on their ranges, (2) evaluate the extent of suitable areas that overlap with existing protected areas and proposed priority areas for biodiversity conservation, and (3) assess how much forest cover still remains in the areas predicted as suitable for the occurrence of these species.
Study area and species
The study area comprises the known distribution area of the three target species, the Caatinga, Cerrado, Amazon and Atlantic Forest biomes, encompassing mainly the north-east of Brazil, but also areas in the north and in the centre-west (Fig. 1). The Atlantic Forest in north-east Brazil is at low altitudes (400–800 m; Tabarelli et al., Reference Tabarelli, Aguiar, Ribeiro, Metzger and Peres2010) with annual rainfall of 1,800–2,000 mm (Rêgo & Hoeflich, Reference Rêgo and Hoeflich2001). The Caatinga and the Cerrado biomes are semiarid environments, with annual precipitation of 250–1,200 mm (Ratter et al., Reference Ratter, Ribeiro and Bridgewater1997; Prado, Reference Prado, Leal, Tabarelli and Silva2003) and 750–2,000 mm (Hunke et al., Reference Hunke, Mueller, Schröder and Zeilhofer2014), respectively. Annual precipitation in the Amazon rainforest biome is 2,000–3,664 mm (Villar et al., Reference Villar, Rnchail, Guyot, Cochonneau, Naziano, Lavado and De Oliveira2009).
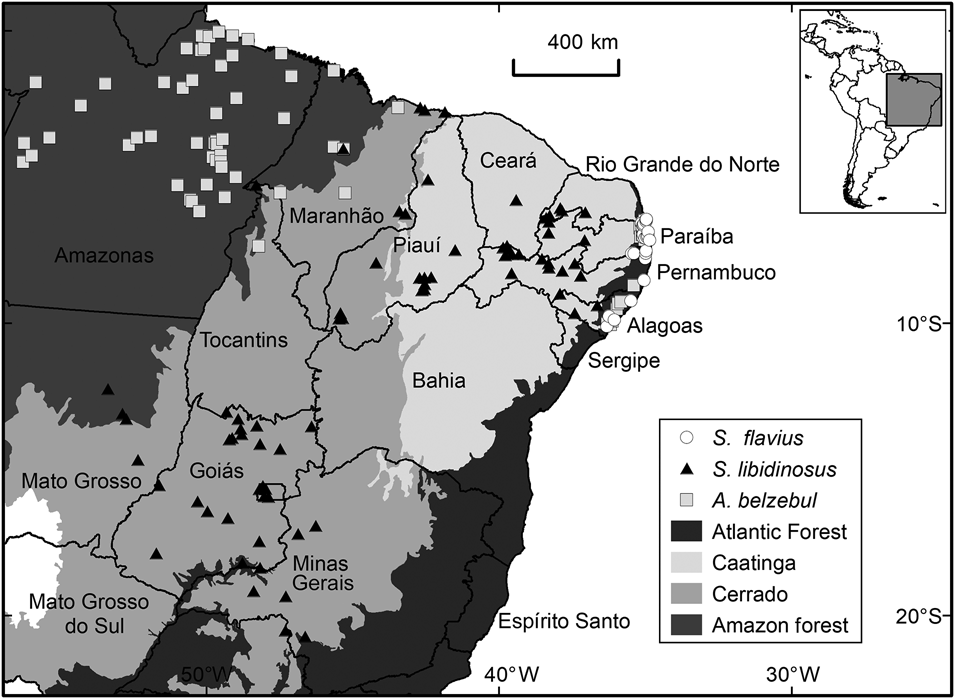
Fig. 1 Occurrence records for Alouatta belzebul, Sapajus flavius and Sapajus libidinosus in the biomes and the Brazilian states included in our study area.
Alouatta belzebul has a disjunct distribution, occurring in the north-eastern Atlantic Forest and lower eastern Amazon in the Brazilian states of Amapá, Pará and Maranhão (Veiga et al., Reference Veiga, Kierulff and de Oliveira2008). It is folivorous–frugivorous (Pinto et al., Reference Pinto, Azevedo-Ramos and de Carvalho2013) and categorized as Vulnerable on the IUCN Red List because its population has declined by 30% over 30 years (Veiga et al., Reference Veiga, Kierulff and de Oliveira2008). It is estimated that the population restricted to the Atlantic Forest has only 200 individuals (Veiga et al., Reference Veiga, Kierulff and de Oliveira2008).
Sapajus flavius occurs in the Atlantic Forest and Caatinga of north-east Brazil (Martins et al., Reference Martins, Valença-Montenegro, Fialho, Laroque and Di Fiore2016; Valença Montenegro et al., Reference Valença Montenegro, Bezerra, Fialho, Jerusalinsky, Lynch Alfaro and Martins2020). This species has a generalist diet (de Souza & Ferreira, Reference de Souza Lins and Ferreira2019; Medeiros et al., Reference Medeiros, Bastos, Jones and Bezerra2019) and is categorized as Endangered on the IUCN Red List because of habitat loss and fragmentation resulting from coastal development and sugar cane plantations (Valença Montenegro et al., Reference Valença Montenegro, Bezerra, Fialho, Jerusalinsky, Lynch Alfaro and Martins2020). It is recognized as one of the most threatened primates globally (Mittermeier et al., Reference Mittermeier, Rylands, Schwitzer, Taylor, Chiozza and Williamson2012), although it is no longer included in the list of the top 25 most endangered primate species (Schwitzer et al., Reference Schwitzer, Mittermeier, Rylands, Chiozza, Williamson and Byler2019).
Sapajus libidinosus inhabits dry forests in semiarid areas, including the Caatinga and Cerrado biomes (Rylands & Kierulff, Reference Rylands and Kierulff2015). Although categorized as Least Concern on the IUCN Red List (Rylands & Kierulff, Reference Rylands and Kierulff2015), the Brazilian government considers it to be Near Threatened (ICMBio, 2016) and it is likely to become more threatened because of habitat loss (Beuchle et al., Reference Beuchle, Grecchi, Shimabukuro, Seliger, Eva, Sano and Achard2015; Rylands & Kierulff, Reference Rylands and Kierulff2015) and illegal pet trade (Nascimento et al., Reference Nascimento, Schiavetti and Montaño2013).
Methods
Species distribution modelling
We obtained occurrence data for the three target species from the Global Biodiversity Information Facility (2017) and speciesLink (2017). We also retrieved location records from the literature using the search terms Sapajus, Sapajus libidinosus, capuchin monkeys, Cebus, Cebus libidinosus, flavius, Cebus flavius, Alouatta, Alouatta belzebul, guariba, bugio, bugio-de-mãos-ruivas, macaco-prego, macaco-prego-galego, macaco-prego-da-caatinga, red-handed howler monkey, howler monkey, blonde capuchin monkey, and bearded capuchin monkey in ScienceDirect (Elsevier, Amsterdam, The Netherlands), Web of Science (Clarivate Analytics, Philadelphia, USA), Periódicos CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasilia, Brazil) and Google Scholar (Google, Mountain View, USA). We also collected new location data for S. libidinosus during 10 expeditions to nine localities in the state of Pernambuco, Brazil, during May 2016–March 2017. All records were validated using Google Earth satellite maps (Google, Mountain View, USA) to exclude records outside forested areas, which were probably the results of inaccurate coordinates.
We generated species distribution models with MaxEnt 3.4.1 (Phillips et al., Reference Phillips, Anderson and Schapire2006). This tool uses a maximum entropy algorithm to select environmental variables that explain species distribution using presence-only data (Phillips et al., Reference Phillips, Anderson and Schapire2006). The background was delimited, for each species, as a buffer of 500 km generated around the minimum convex polygon of all known occurrence records (Supplementary Fig. 1). We obtained climatic variables from WorldClim 1.4 (Hijmans et al., Reference Hijmans, Cameron, Parra, Jones and Jarvis2005), Brazil ecoregions (MMA, 2003) and geomorphology databases (INPE, 2001), and generated the slope layer from the altitude layer (Diva-GIS, 2011). To avoid collinearity, we only included variables that were not highly correlated (r < 0.8; Supplementary Table 1). Through principal component analysis (Supplementary Table 2) we selected the most important variables to include in each species' model. These variables explained 80% of the distribution models. All variables were downloaded at (or converted to) a resolution of 30 arc-seconds (c. 1 km2), which was the cell size for the analyses, using ArcGIS 10.1 (Esri, Redlands, USA).
We reduced spatial autocorrelation among location records through an environmental heterogeneity rarefaction analysis using SDMTools box (Brown, Reference Brown2014) in ArcGIS. We created a buffer of 10 km around each occurrence record and randomly removed duplicate points within the zones of the buffers. We retained records that were within the same buffer but in pixels with different environmental characteristics. This procedure was performed to avoid a sampling bias, whereby clusters tend to give greater weight to environmental variables (Renner et al., Reference Renner, Elith, Baddeley, Fithian, Hastie and Phillips2015).
Models were projected into the future (2070) based on 13 general circulation models used in the 5th Intergovernmental Panel on Climate Change (IPCC) report (Flato et al., Reference Flato, Marotzke, Abiodun, Braconnot, Chou, Collins, Stocker, Qin, Plattner, Tignor, Allen, Boschung, Nauels, Xia, Bex and Midgley2013): ACCESS1-0, HadGem2-ES, Miroc-ESM, BCC-CSM1-1, CCSM4, CNRM-CM5, GFDL-CM3, GISS-E2-R, INMCM4, IPSL-CM5A-LR, MPI-ESM-LR, MRI-CGCM3 and NorESM1-M. We selected the 2070 scenarios, which predict climate towards the end of the century, because predictions based on shorter time frames would not provide an adequate parameter representation of the impact of climate change on the species. We considered two representative concentration pathways, defined by the trajectory of greenhouse gas emissions and subsequent radiative forcing (Wayne, Reference Wayne2013): 4.5 W/m2 (moderate climate change scenario) and 8.5 W/m2 (severe climate change scenario). We converted the continuous model output into binary maps using the thresholding method, which maximizes the sum of sensitivity and specificity (Liu et al., Reference Liu, White and Newell2013). To incorporate model variability while avoiding biases resulting from outlier model outputs we generated the final future maps for each scenario based on agreement between > 75% of the Maxent general circulation models outputs (upper quantile). This was done using ArcGIS, by adding the binary model outputs generated from the 13 general circulation models and reclassifying the resulting map, assigning a value of 0 (unsuitable) to cells that were identified as suitable by no more than three models, and 1 (suitable) to cells identified as suitable by more than three models.
We ran models with 1,500 interactions using the cloglog model output. We used the ENMeval package in R 3.4.3 (R Core Team, 2017) to evaluate and select the best model parameterization (regularization multiplier value and number of parameters) based on the Akaike information criterion corrected for small sample sizes (AICc; Muscarella et al., Reference Muscarella, Galante, Soley-Guardia, Boria, Kass, Uriarte and Anderson2014). The best fit model included three features (linear, quadratic and hinge) and a regularization multiplier of 1. We evaluated the performance of the models using 10-fold cross-validations and the area under the receiver operator curve (AUC), a measure of the ability of the model to distinguish between presence locations and background/pseudo-absences. We compared model AUC scores with 100 null models, generated through resampling the isothermality layer in ENMTools (Warren et al., Reference Warren, Glor and Turelli2010), to determine whether our models performed significantly better than random (Raes & ter Steege, Reference Raes and ter Steege2007).
Gap analysis
We carried out gap and range change analyses using the reclassified binary maps. We calculated the representation of predicted current and future suitable areas for each species by overlaying in ArcGIS the outputs of our models with maps of Brazilian protected areas (ICMBio, 2017; MMA, 2018b), priority areas for biodiversity conservation (MMA, 2018a) and forest cover (IBGE, 2017). To identify protected areas and priority areas that will retain climatic suitability in the future, we overlapped areas that were predicted to be suitable under both present and future conditions with existing protected areas and priority areas. We overlapped priority areas and protected areas with our modelled suitable areas to identify relevant locations for the expansion or creation of protected areas. We also identified protected areas that are likely to be under threat because of hunting and other anthropogenic impacts by overlapping model outputs with a human settlement map (IBGE, 2017).
To categorize the degree of protection of the areas predicted by our models to be suitable for the target species, we considered the following categories: high protection status (protected areas of integral protection, where human settlement is not permitted, but certain activities such as scientific research and ecotourism are); medium protection status (areas under permanent protection in which sustainable use of natural resources and human occupation are permitted); low protection status (protected areas in which human settlement and development are permitted as well sustainable use of natural resources); and unprotected (areas not formally protected).
Areas defined by the Brazilian government as priority areas for biodiversity conservation are areas where conservation efforts should be directed for the planning and implementation of actions such as the creation of protected areas, licensing, inspection, and promotion of sustainable use. We considered areas occupied by forests as those with trees > 5 m tall, including areas of dense, open, seasonal and mixed ombrophilous forest, as well as forested savannah, forested campinarana and mangroves (IBGE, 2017). We defined human settlements as areas characterized by urban use, structured by buildings and road systems, where non-agricultural artificial surfaces predominate (IBGE, 2017). This category includes cities, towns, roads, services and transport, power grids, communication infrastructure and associated land, areas occupied by industrial and commercial complexes, buildings (which may in some cases be located in periurban areas), Indigenous villages and mining areas.
Results
We found 223 occurrence records for the three target species, 176 of which were retained after validation. These comprised 66 records of A. belzebul, 33 of S. flavius and 77 of S. libidinosus (Supplementary Table 3).
All species distribution models were able to discriminate between true presence and pseudo-absences (AUCtest range: 0.82–0.97; Table 1) and performed better than null models because they fell outside the range of AUC values generated from 100 null models (AUCtrain range: 0.61–0.80). Geomorphology, annual precipitation and ecoregion were the main environmental variables affecting habitat suitability for the target species (Table 1). The model considering current conditions predicted suitable areas of 671, 47,184 and 1,059,360 km2 for A. belzebul, S. flavius and S. libidinosus, respectively (Table 2, Fig. 2). Our future models considering climate change predicted a reduction in the areas suitable for all species (Table 2, Fig. 3).
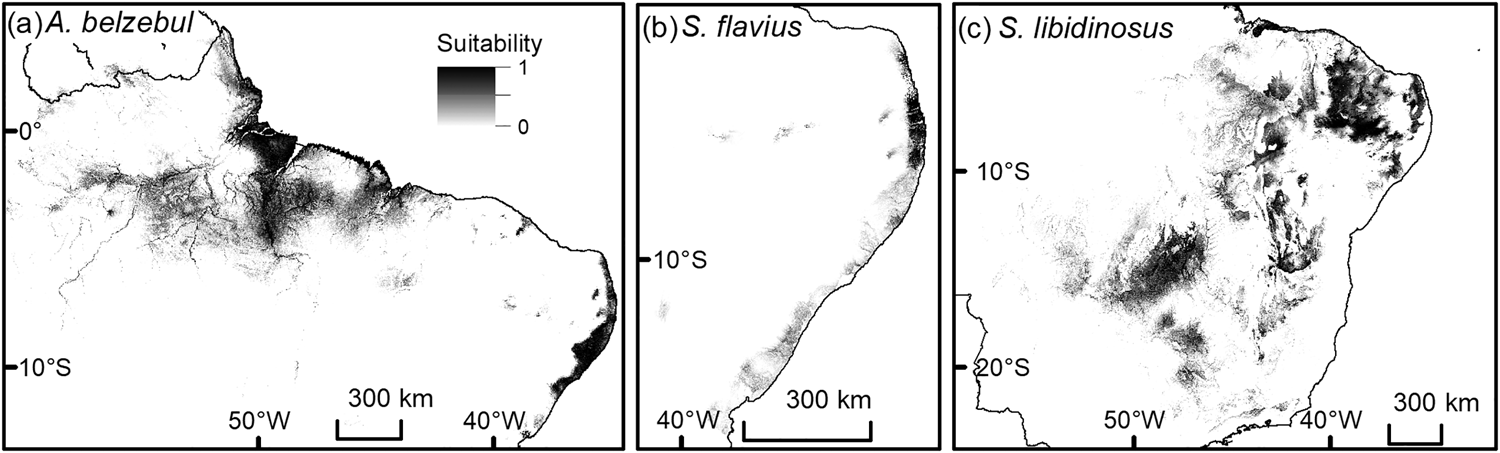
Fig. 2 Predicted current distribution of suitable areas for the occurrence of (a) A. belzebul, (b) S. flavius and (c) S. libidinosus. Suitability ranges from low (0) to high (1).
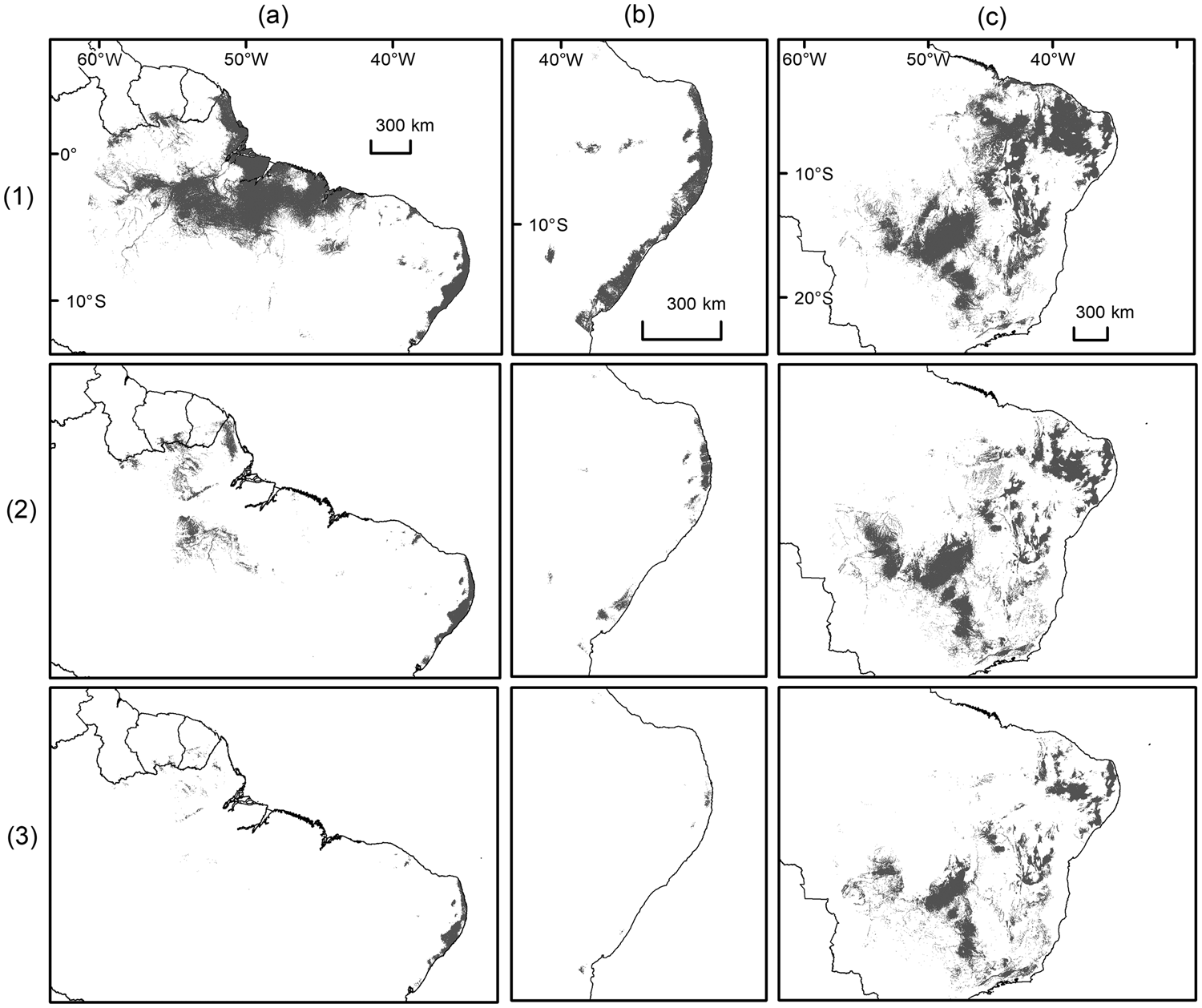
Fig. 3 Binary maps of present and future predictive distribution for (a) A. belzebul, (b) S. flavius and (c) S. libidinosus, under (1) current conditions, and future conditions under scenarios of (2) moderate (2070, RCP 4.5) and (3) severe (2070, RCP 8.5) climate change. Future predictions are based on 13 general circulation models for each scenario. Shaded areas indicate predicted suitable habitats above the maximum training sensitivity plus specificity threshold.
Table 1 Results of the species distribution models for the three studied primates, number of location records included in the models (N), results of the statistical tests used to evaluate model discrimination ability (area under the receiver operator curve; AUC) for training and test datasets and the per cent contribution of the different environmental variables.

1Bio2, mean diurnal range (mean of the difference of the monthly maximum and minimum temperatures over 1 year); Bio3, isothermality; Bio8, mean temperature of wettest quarter; Bio11, mean temperature of coldest quarter; Bio12, annual precipitation; Bio15, precipitation seasonality; Bio18, precipitation of warmest quarter; Eco, ecoregion; Geom, geomorphology.
Table 2 Area predicted to be suitable for the target species (km2), considering current conditions, and future (2070) conditions moderate (RCP 4.5) and severe (RCP 8.5) climate change emission scenarios, and including their geographical range and future range loss.
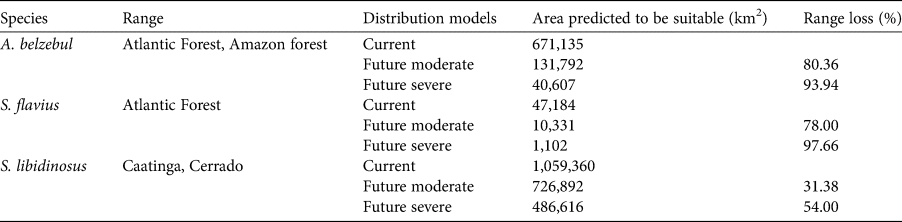
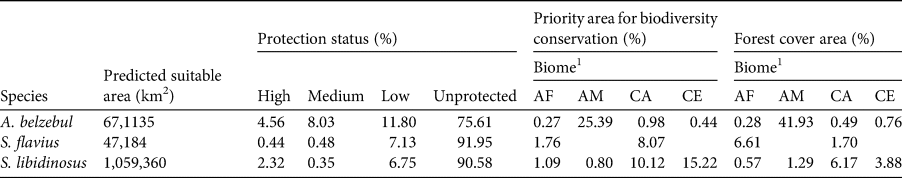
Gap analysis showed that only 24, 8 and 9% of the areas predicted to be suitable under current climatic conditions for A. belzebul, S. flavius and S. libidinosus, respectively, fall within existing protected areas (Table 3, Fig. 4), and 72% of these areas are of low protection status. Approximately 88% of the areas predicted to be suitable are unprotected.
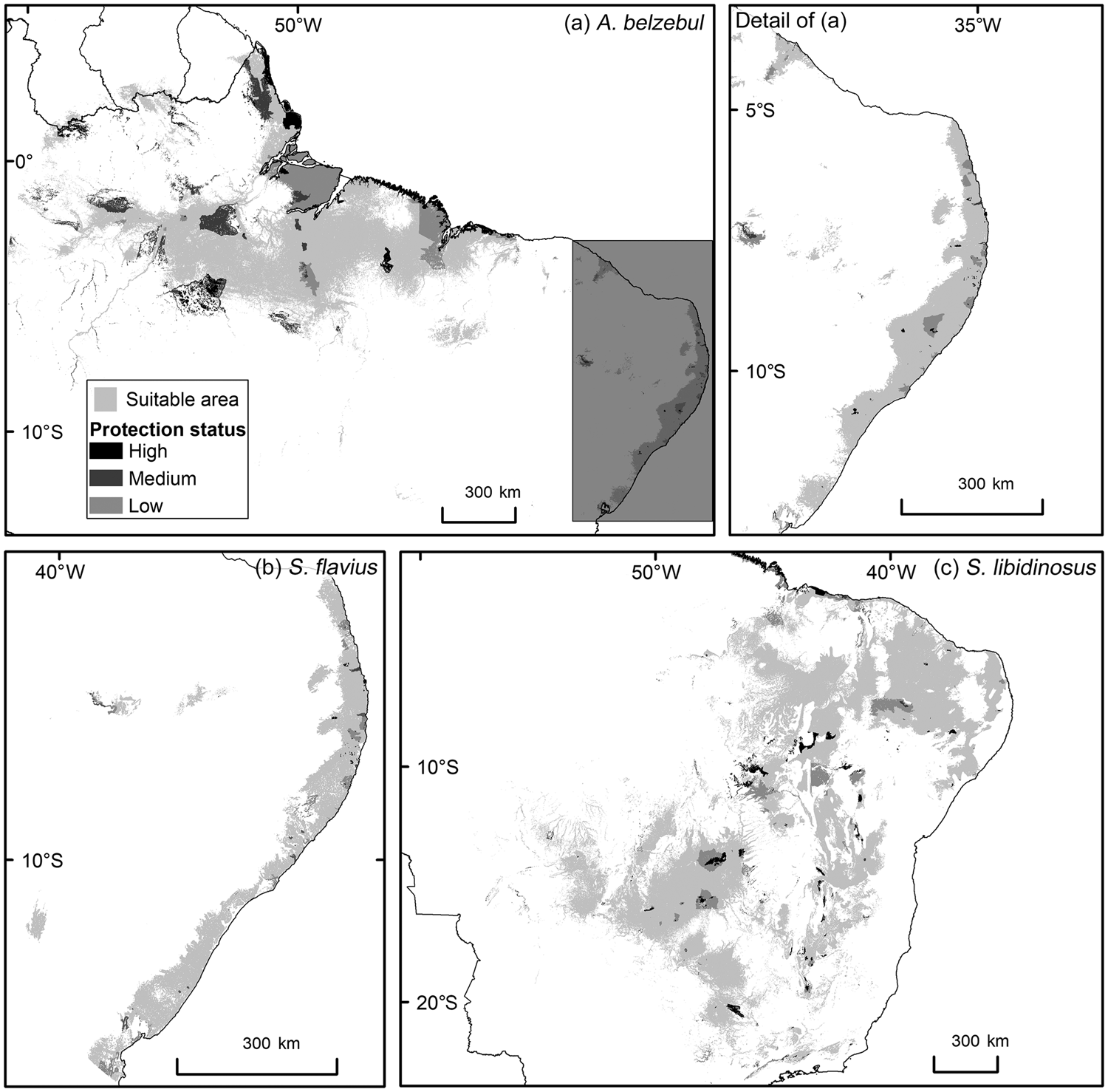
Fig. 4 Predicted suitable areas for the occurrence of (a) A. belzebul, (b) S. flavius and (c) S. libidinosus, and their overlap with protected areas of high, medium and low protection status.
Table 3 Area predicted to be suitable for occurrence of the three primates and their representation in areas with different protections status (high, medium, low and unprotected), in priority areas for biodiversity conservation and in areas with forest cover.
1Biomes: AF, Atlantic Forest; AM, Amazon forest; CA, Caatinga; CE, Cerrado.
In our models, we overlapped areas predicted to be suitable for the occurrence of the three target species with government priority areas and forest cover layers. We found that 27% of the suitable areas for all three target species together fall within government priority areas for conservation (10% in the Amazon forest, 10% in the Cerrado, 6% in the Caatinga and 1% in the Atlantic Forest). Only 24% of the suitable areas are currently forested (17% in the Amazon forest, 4% in the Caatinga, 2.5% in the Cerrado and 0.5% in the Atlantic Forest; Table 3). Binary maps of current predictive distribution with their priority areas and forest cover are detailed in Supplementary Figs 2 and 3, respectively.
To identify relevant areas for the expansion or creation of protected areas, and potential connectivity between suitable areas, we overlapped government priority areas and protected areas with our modelled suitable areas. We found that 96, 99 and 74% of the government priority areas within A. belzebul, S. flavius and S. libidinosus suitability areas, respectively, are outside protected areas (Supplementary Table 4).
Our analysis showed that 23% (93), 11% (43) and 73% (295) of protected areas will maintain climatic suitability under the moderate future climate change scenario for A. belzebul, S. flavius and S. libidinosus, respectively. These numbers decrease to 12% (50), 3% (13) and 67% (270) when we consider the more severe future scenario (see Supplementary Table 5 for a list of protected areas). Of all the protected areas identified as climatically suitable for the three primate species under present and future conditions, 14% (56) overlap with human settlements. For S. flavius, in particular, 33% of climatically suitable protected areas overlap with human settlements (Supplementary Table 5). We also identified that 18% (91), 2% (12) and 88% (441) of government priority areas will remain climatically suitable under the moderate future climate change scenario for A. belzebul, S. flavius and S. libidinosus, respectively. These numbers decrease to 5% (25), 0.4% (2) and 61% (357) when we consider the more severe future scenario (see Supplementary Table 4 for a list of government priority areas and conservation actions for each area).
Discussion
Our findings indicate concern for the future of A. belzebul, S. flavius and S. libidinosus, as 88% of the areas predicted to be suitable for these species are unprotected. The remaining 12% of the suitable areas fall within protected areas, but 72% of these have low protection status. Only 27% of the overall areas predicted to be suitable for the three species overlap with priority areas for conservation, and only 24% are currently forested. Future models predict a near total loss of climatic suitability for the three species in tropical forests (Amazon and/or Atlantic Forest) and loss of a quarter of suitable areas in the semiarid regions (Caatinga and Cerrado).
Our models were able to distinguish between the habitats of the three species. We found that habitat suitability is affected by geomorphology, annual precipitation and ecoregion, which together influence vegetation structure and composition (Shi-kui et al., Reference Shi-kui, Weia, Xu-kunb, Yongc, Shuaia and Xiaoxaia2019). Characteristics such as temperature variation and deciduous and semideciduous vegetation differentiate the species’ habitat needs from those of other primates (Ratter et al., Reference Ratter, Ribeiro and Bridgewater1997; Prado, Reference Prado, Leal, Tabarelli and Silva2003). Water availability and changes in soil composition also play an important role by controlling the type of vegetation that can grow in different landscapes (El-Keblawy et al., Reference El-Keblawy, Abdelfattah and Khedr2015; Cowles et al., Reference Cowles, Boldgiv, Liancourt, Petraitis and Casper2018).
Our findings reflect the current situation of the protected areas system in Brazil, with a large proportion categorized as low protection status. Currently, only 10% of the Atlantic Forest is under protection and only 2.6% falls within the high protection status (MMA, 2018b). There is even less protection for the Caatinga and Cerrado biomes, with 8% of each protected and only 1.6 and 3%, respectively, within the integral protection areas (MMA, 2018b). In contrast, 28% of the Amazon falls within protected areas and 9% within integral protection areas (MMA, 2018b). In areas with low protection status, extractive activities are permitted in accordance with applicable law, resulting in inadequate protection for species at imminent risk of extinction (Schulze et al., Reference Schulze, Knighs, Coad, Geldmann, Leverington and Eassom2018). In addition, some areas officially designated as protected lack essential infrastructure or resources, resulting in little if any actual protection (Saout et al., Reference Saout, Hoffmann, Shi, Hughes, Bernard and Brooks2013; Oliveira & Bernard, Reference Oliveira and Bernard2017). Despite problems related to the poor management of these areas, protected areas are relevant because they prevent the conversion of natural ecosystems (Geldmann et al., Reference Geldmann, Barnes, Coad, Craigie, Hockings and Burgess2013) and support a greater diversity and abundance of species than unprotected areas (Gray et al., Reference Gray, Hill, Newbold, Hudson, Börger and Contu2016).
We observed the least overlap between habitat suitability for the primate species and government priority areas for biodiversity conservation in the north-eastern Atlantic Forest. This is because most of the Atlantic Forest priority areas are concentrated in southern Brazil (MMA, 2018a). Only two government priority areas were considered in north-east Brazil and these are located in the state of Bahia, in areas where the target species do not occur. Although government priority areas cover c. 25% of biomes such as the Amazon, Caatinga and Cerrado, only 6–10% of these areas were predicted as suitable for the three primate species in our study. Nevertheless, because a large proportion of government priority areas within the modelled suitable areas is not within protected areas, it will be necessary to identify potential connectivity areas, and areas for the expansion or creation of new protected areas, to conserve these primate species. The government priority areas within the areas suitable for the target species that will remain suitable under future climate change (Supplementary Table 4) are potential sites for reintroducing confiscated individuals from the illegal wildlife trade, provided these areas are under some level of legal protection.
The low forest cover in areas predicted to be suitable for the three target species is mainly a result of the high anthropogenic impact on biomes such as the Atlantic Forest, Caatinga and Cerrado. The north-eastern Atlantic Forest is highly fragmented (Ribeiro et al., Reference Ribeiro, Martensen, Metzger, Tabarelli, Scarano, Fortin, M.-J., Zachos and Habel2011), and in the Pernambuco Endemism Center, which includes the distribution of A. belzebul and S. flavius, 99% of the remnant forest fragments are smaller than 50 ha (Silva & Fialho, Reference Silva and Fialho2013). Similarly, only 50% of the original vegetation remains in the Caatinga and Cerrado (MMA, 2016; Strassburg et al., Reference Strassburg, Brooks, Feltran-Barbieri, Iribarrem, Crouzeilles and Loyola2017). In addition, habitat remnants within the Atlantic Forest and semiarid zones are surrounded by an inhospitable agricultural matrix that presents a barrier to movement and dispersal (Portillo-Quintero & Sánchez-Azofeifa, Reference Portillo-Quintero and Sánchez-Azofeifa2010; Ribeiro et al., Reference Ribeiro, Martensen, Metzger, Tabarelli, Scarano, Fortin, M.-J., Zachos and Habel2011). Areas of occurrence of S. flavius are also affected by mining (e.g. Bezerra et al., Reference Bezerra, Bastos, Souto and Jones2014). Although some primate species are able to cross and benefit from non-forested matrices, such as agricultural areas (Mandujano et al., Reference Mandujano, Escobedo-Morales and Palacios-Silva2004; Canale et al., Reference Canale, Kierulff, Chivers, Marsh and Chapman2013; Souza-Alves et al., Reference Souza-Alves, Mourthe, Hilário, Bicca-Marques, Rehg and Gestich2019), this brings them into closer contact with people, potentially increasing negative human–wildlife interactions. Primates are threatened by dogs, contact with electrical wires, the illegal pet trade and retaliation from farmers because of crop use (Fuentes, Reference Fuentes2006).
Our data corroborate other studies suggesting that both the Amazon and the Atlantic Forest will suffer significant biodiversity losses as a result of the combined effect of deforestation and climate change (Pires & Costa, Reference Pires and Costa2013; Bellard et al., Reference Bellard, Leclerc, Leroy, Bakkenes, Veloz, Thuiller and Courchamp2014). In the Amazon, deforestation can alter the balance of the forest and transform it into a savannah environment (Costa & Pires, Reference Costa and Pires2010). Future climate change projections indicate a 20% increase in aridity, both for the Amazon rainforest and for the north-east of Brazil (Franchito et al., Reference Franchito, Fernandez and Pareja2014), and an increase in temperature (Marengo et al., Reference Marengo, Torres and Alves2017). Biodiversity hotspots, such as the Cerrado and the Atlantic Forest, are predicted to be adversely affected by future climate change and to lose c. 25% of their endemic species (Bellard et al., Reference Bellard, Leclerc, Leroy, Bakkenes, Veloz, Thuiller and Courchamp2014) because they are projected to become arid lands (Costa & Pires, Reference Costa and Pires2010; Franchito et al., Reference Franchito, Fernandez and Pareja2014). It is believed that there will be changes in land use because of the projected decrease in herbaceous vegetation in the Cerrado and an increase in the extent of fragmentation and conversion to pasture in the Atlantic Forest (Bellard et al., Reference Bellard, Leclerc, Leroy, Bakkenes, Veloz, Thuiller and Courchamp2014). Changes in spatial configuration and habitat quality may affect the distribution and density of primate species (Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque and Di Fiore2017), as well as the quantity and quality of food resources available to them (Dunn et al., Reference Dunn, Cristóbal-Azkarate and Vea2009; Morellato et al., Reference Morellato, Alberton, Alvarado, Borges, Buisson and Gabriela2016).
To suggest important areas for conservation action, we identified protected areas that will maintain climate suitability in the future and may serve as a refuge for the target species. These areas were well represented in the areas suitable for S. libidinosus, but much less so in the areas suitable for A. belzebul and S. flavius. This indicates the importance of expanding or creating new protected areas for the latter two species, especially in the north-east Atlantic Forest. Although the overall percentage of protected areas affected by urban settlements was relatively low in our study (c. 14%), a larger proportion of the areas suitable for S. flavius was affected by human settlements (c. 33%). Human settlements close to areas suitable for these primates may result in hunting, but no data are available on current hunting pressure on S. flavius. Overexploitation has already eradicated several primate populations in Brazil, including populations of capuchin monkeys (Estrada et al., Reference Estrada, Garber, Mittermeier, Wich, Gouveia and Dobrovolski2018). Strengthening environmental policies and enforcing laws is key to preventing hunting and further deforestation (Brancalion et al., Reference Brancalion, Garcia, Loyola, Rodrigues, Pillar and Lewinsohn2016; Estrada et al., Reference Estrada, Garber, Mittermeier, Wich, Gouveia and Dobrovolski2018).
Our study highlights concern regarding the conservation of Neotropical primates in general. We provide important information for the conservation of three primate species, two of which are part of the Brazilian Action Plan for the Conservation of Primates in Northeast Brazil. Although the three target species inhabit areas considered to be of global conservation importance (Brooks et al., Reference Brooks, Mittermeier, da Fonseca, Gerlach, Hoffmann and Lamoreux2006), the areas predicted to be suitable for these species are mostly outside protected areas and have low forest cover, especially in the Atlantic Forest. The Brazilian government has designated priority areas for conservation, but these are not sufficient to maintain the primate populations we have studied. By determining suitable areas for the occurrence of the target species under present and future conditions we have identified areas where conservation efforts should focus, to reduce habitat destruction and fragmentation. Creating and maintaining protected areas could help to preserve forested areas and contribute to the survival of these species.
Our models show that future climate change could lead to substantial range losses for the three primate species. This is of concern as these changes can affect the establishment of populations and their ability to survive in these areas in the long term. Our findings will facilitate assessment of the conservation status of each species and the establishment of goals in action plans for the conservation of other primate species inhabiting the same regions. We will share our findings and recommendations with key stakeholders such as the Centro Nacional de Pesquisa e Conservação de Primatas Brasileiros from the Chico Mendes Institute for Biodiversity Conservation, an administrative arm responsible for primate conservation action plans in Brazil. The sharing of our impartial scientific study may help such institutions plan future conservation actions, considering that the current Brazilian government is weakening environmental laws, leading to further deforestation, fragmentation and destruction of wildlife habitats in the country (Ferrante & Fearnside, Reference Ferrante and Fearnside2019).
Acknowledgements
We thank Felipe França, José da Silva, Silvanete da Silva, Antônio Alencar Sampaio, Guga do Pinheiro, João da Silva and Yuri Marinho for assistance with fieldwork, Agência Estadual de Meio Ambiente in the Pimenteira State Park, the Parnamirim Irrigated Agriculture Station—UFRPE and the Programa de Pós-graduação em Biologia Animal (Graduate Programme in Animal Biology) for logistical support. We thank Enrico Bernard, Maria Adelia Oliveira and João Lucas Feitosa for fruitful discussions on earlier stages of this study. We acknowledge funding to BLCM from Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco (FACEPE; grant number: IBPG-1013-2.04/14) and Coordination for the Improvement of Higher Education Personal (CAPES; grant number: PDSE 88881.134891/2016-01); to JPS-A from CAPES (grant number: 527091) and FACEPE (grant numbers: BCT-0025-2.05/17; BFP0149-2.05/10); to OR through a Natural Environment Research Council Independent Research Fellowship (NE/M018660/1). This study is a new contribution from the blonde capuchin research and conservation project supported by the Mohamed bin Zayed Species Conservation Fund, Margot Marsh Biodiversity Foundation, Rufford Small Grant Foundation, FACEPE (grant numbers: APQ-1534-2.04/10; APQ-0143-2.04/14; BFT-0160-2.04/17; BFT-0014-2.05/20) and Brazilian National Council for Scientific and Technological Development (CNPq; grant numbers: Universal 445071/2014-1; Pq2 309256/2019-4).
Author contributions
Study design: BM, BB; fieldwork: BM; data analysis and writing: all authors.
Conflicts of interest
None.
Ethical standards
This research abides by the Oryx guidelines on ethical standards and was carried out in compliance with Brazilian law (fieldwork licence: SISBIO/ICMBio—52404-1; license for interviews: Plataforma Brasil—CAAE-49198215.3.0000.5208/Approval-1.266.360).











