Introduction
Dogs are the oldest companion animals of humans, and we share over 60 of the >400 infectious diseases that dogs can carry (Macpherson et al., Reference Macpherson, Pinckney, Sylvester, Bidaisee and Macpherson2022). These include zoonotic gastrointestinal parasites which are often of significant public health importance. In tropical regions of the world, such as in Grenada, West Indies, some of these have been reported, including Ancylostoma caninum and A. ceylanicum (Zendejas-Heredia et al., Reference Zendejas-Heredia, Colella, Macpherson, Sylvester, Gasser, Macpherson and Traub2022). Dogs are the main reservoir hosts for these zoonotic parasites, due to their free roaming behaviour, uncontrolled reproduction and limited access to veterinary care (Lyons et al., Reference Lyons, Malhotra and Thompson2022).
Ancylostoma ceylanicum and Strongyloides stercoralis are the only 2 known canine zoonotic soil transmitted helminths (STHs) that result in patent infections in humans (Schar et al., Reference Schar, Trostdorf, Giardina, Khieu, Muth, Marti, Vounatsou and Odermatt2013, Bradbury et al., Reference Bradbury, Hii, Harrington, Speare and Traub2017). Currently, A. ceylanicum is recognized as the second most common species of hookworm infecting humans in the Asia Pacific region, comprising between 6–23% of all intestinal hookworm infections in people living in endemic areas (Traub et al., Reference Traub, Zendejas-Heredia, Massetti and Colella2021). Ancylostoma ceylanicum and A. caninum can cause cutaneous larva migrans (CLM) and intestinal symptoms in humans, and haemorrhagic diarrhoea and chronic iron deficiency anaemia in dogs (Traub et al., Reference Traub, Zendejas-Heredia, Massetti and Colella2021). The global prevalence of strongyloidiasis was estimated at 8.1% in 2017, with 76% of cases recorded in Southeast Asia, Africa and the Western Pacific (Buonfrate et al., Reference Buonfrate, Bisanzio, Giorli, Odermatt, Furst, Greenaway, French, Reithinger, Gobbi, Montresor and Bisoffi2020). Strongyloides spp. have the potential for transmission to humans and other animals through the parasite's ability to reproduce in the environment – a unique strategy amongst parasitic helminths of mammals (Jaleta et al., Reference Jaleta, Zhou, Bemm, Schar, Khieu, Muth, Odermatt, Lok and Streit2017). Strongyloides spp. infections in both dogs and humans are often asymptomatic but can become fatal together with chronic comorbidities (Streit, Reference Streit, Strube and Mehlhorn2021). Toxocara canis is estimated to infect >100 million dogs worldwide (Rostami et al., Reference Rostami, Riahi, Hofmann, Ma, Wang, Behniafar, Taghipour, Fakhri, Spotin, Chang, Macpherson, Hotez and Gasser2020), with majority of puppies becoming infected through vertical transmission (Schwartz et al., Reference Schwartz, Bidaisee, Fields, Macpherson and Macpherson2022). Nonetheless older dogs can harbour patent infections as a consequence of a compromised immune status (Fahrion et al., Reference Fahrion, Staebler and Deplazes2008). Humans are often asymptomatic but can develop visceral larva migrans, ocular larva migrans, neurotoxocariasis and covert toxocariasis (Macpherson, Reference Macpherson2013). A recent seroprevalence study showed that 37% (93/253; 95% CI 36–38) of 253 people in the local population were seropositive for T. canis using an IgG-based ELISA test (Ziegler, Reference Ziegler2022, unpublished results), suggesting that humans are exposed to this parasite in Grenada. While initial treatment of puppies with an anthelmintic has been recommended at 2 weeks postpartum for decades, puppies in Grenada have been shown to already be shedding eggs at this time (Schwartz, et al., Reference Schwartz, Bidaisee, Fields, Macpherson and Macpherson2022).
There is a paucity of information on STHs in the Caribbean, including Grenada, which represents a major gap in the knowledge of the transmission and public health relevance of these parasites in this region. Thus, assessing factors involved in the transmission of these helminths should aid in developing targeted control programmes using a One Health approach (Walker et al., Reference Walker, Lambert, Neves, Worsley, Traub and Collela2023).
Here, combined microscopic and molecular tools were used to estimate the prevalence and to molecularly identify nematodes affecting dogs in Grenada, and a multiple logistic regression model was used to assess risk factors associated with polyparasitism in these dogs.
Materials and methods
Ethics approval
This project was approved by the Institutional Animal Care and Use Committee (IACUC) of the St. George's University, Grenada, West Indies (approval code: IACUC-21001-R).
Study area and population
Grenada is part of the Windward Islands in the Caribbean and has a land area of 348.5 km2. The human and dog populations are ~110 000 and 35 000, respectively (Catan and Macpherson, Reference Catan, Macpherson and Bekoff2007).
In May 2021, fecal samples were obtained from community-owned and pet dogs throughout all 6 parishes, mainly from the southern half of the country. Many of these were Pothounds, which are nondescript, medium–sized dogs found throughout the Caribbean and other tropical areas (Catan and Macpherson, Reference Catan, Macpherson and Bekoff2007). Pothounds are often loosely owned within a community and roam freely. They usually do not have a history of previous veterinary examination and are not regularly given drugs to control endo- or ecto-parasites (Schwartz et al., Reference Schwartz, Bidaisee, Fields, Macpherson and Macpherson2022).
Sample collection
Most dogs in Grenada have a primary owner, even if they roam and are largely ‘community-owned’. This person was the point of contact for each dog. Following informed consent from dog owners, a physical examination was performed before fecal collection to record the body condition score (BCS) and general health status (e.g. anaemia or pregnancy status) of each dog sampled. Age was assessed from owner knowledge and also looking at age related changes such as the state of a dog's teeth. The American Animal Hospital Association (2010) 9 point body condition scoring system was used to guide BCS assignment for dogs enrolled in this study. Pregnancy was assessed upon history taking and physical examination and confirmed by a veterinarian using a portable ultrasound. Anaemia was assessed through mucous membrane colour. All physical examinations were performed by a third-year veterinary student under the supervision of an experienced clinical veterinarian. A minimum of 1–3 grams of feces was collected, except for young puppies where less than 1 g was taken. Fecal samples were obtained rectally or an aliquot was taken from the top of a fresh fecal pile immediately following observation of defecation. Each sample was accompanied by a written record of the date and time of sample collection, a name or description of identifying features of the dog, their sex and age, the village and parish of origin, whether it was allowed to roam or kept indoors, any history of preventative drug use, ectoparasites detected, and ‘owner’ details, including name and phone number. This information was stored in an Excel spreadsheet. Fecal samples were placed into individual sealed and labelled plastic tubes and stored in an insulated container containing ice packs. Samples were carried for 1–10 h during field collection before arriving in the laboratory, after which they were kept at 4°C. Aliquots (~1 g) of individual fecal samples were fixed in 70% ethanol and sent to The University of Melbourne (Australia) for the identification of species of STHs by multiplex qPCR (methodology described below).
Microscopic examination of fecal samples
A conventional passive fecal flotation method (Dryden et al., Reference Dryden, Payne, Ridley and Smith2005) was used for the detection of eggs. In brief, 0.5–1 gram of each fecal sample was homogenized in zinc sulphate solution (specific gravity: 1.2) in an Ovassay Plus fecal flotation device (Zoetis, USA) and a coverslip placed on the meniscus. After 15 min, the coverslip was lifted, inverted and placed on to a glass slide and examined at 10-times and 40-times magnification using light microscopy (Olympus CH30, USA). The presence and number of nematode eggs visualized under each entire cover slip was recorded.
Extraction of DNA from fecal samples
DNA was extracted from aliquots of individual fecal samples (n = 220; ~200 mg each; following the removal of ethanol and sample rehydration) using a QIAmp® PowerFecal Pro DNA Kit (Qiagen, Germany) according to the manufacturer's instructions with slight modifications: (i) the cell lysis step was performed using 800 μL of solution CD1 employing a FastPrep-24™ 5 G homogeniser (MP Biomedicals); and (ii) DNA was eluted twice using 50 μL of solution C6, the 2 eluates combined and the samples then stored at −20°C until further processing.
Multiplex qPCR
Individual DNA samples were tested (in duplicate) using multiplex Taq-Man probe-based qPCR (M-qPCR) assays to detect a partial region of the first internal transcribed spacer of nuclear rDNA region (ITS-1) of A. caninum, A. ceylanicum, A. braziliense and U. stenocephala (Massetti et al., Reference Massetti, Colella, Zendejas, Ng-Nguyen, Harriott, Marwedel, Wietholeter and Traub2020, Zendejas-Heredia et al., Reference Zendejas-Heredia, Colella, Macpherson, Sylvester, Gasser, Macpherson and Traub2022), a partial region of the 18S rRNA gene of Strongyloides spp. (Verweij et al., Reference Verweij, Canales, Polman, Ziem, Brienen, Polderman and Van Lieshout2009), and a partial region of the ITS-2 for T. canis (Durant et al., Reference Durant, Irenge, Fogt-Wyrwas, Dumont, Doucet, Mignon, Losson and Gala2012). Equine herpes virus (EHV4) was used as an internal control within each PCR, and a mammalian target of a 92 bp–region of the mitochondrial 16S rRNA gene of mammals was employed as a DNA–extraction control (Table 1). M-qPCR reactions were performed as described previously using a modification of the Quantinova Probe PCR (Qiagen, Germany) conducted in a final volume of 10 μL. The cycling conditions were 95°C for 2 min, followed by 40 cycles at 95°C for 15 sec and at 60°C for 1 min using the QIAquant 96x real-time PCR instrument (Qiagen, Germany). Synthetic DNA fragments (gBlocks Gene Fragments, IDT Technologies, Skokie, Illinois, USA) were used as positive controls, and a no-template (negative) control was included in each PCR run.
Table 1. Oligonucleotides, probe sequences and cycling conditions used for the multiplex qPCR reactions for differentiation of hookworm species and detection of Strongyloides spp.

Conventional PCR and DNA sequencing
Samples shown to be test-positive for Strongyloides and T. canis by qPCR were subjected to conventional PCR (cPCR) and DNA sequencing for specific identification. A ~255 bp region of the nuclear 18S rRNA hypervariable region IV was selected as target to identify Strongyloides using the primers New_HVR_IV F (5’-CGGGCCGGACACTATAAGG-3’) and New_HVR_IV R (5’-ATCTCTAAACAGGAACATAATGATCACTAC-3) (Barratt et al., Reference Barratt, Lane, Talundzic, Richins, Robertson, Formenti, Pritt, Verocai, Nascimento de Souza, Mato Soares, Traub, Buonfrate and Bradbury2019). A ~300 bp partial region of the nuclear ITS-2 rRNA gene was selected to identify T. canis (Li et al., Reference Li, Lin, Chen, Sani, Song and Zhu2007). using primers YYI (5′-CGGTGAGCTATGCTGGTGTG-3′) and NC2 (5′-TTAGTTTCTTTTCCTCCGCT-3) (Li et al., Reference Li, Lin, Chen, Sani, Song and Zhu2007) The PCR conditions were 95°C for 5 min, followed by 40 cycles at 94°C for 30 sec, 63°C (Strongyloides spp.) or 56°C (T. canis) for 30 sec, 72°C 30 sec, followed by a final extension at 72°C for 5 min. The cPCR was conducted using HotStartTaq Plus DNA Polymerase (Qiagen, DEU) employing a SimpliAMP Thermal Cycler (Thermo Fisher Scientific, US). PCR products were examined on a 1.5% (w/v) agarose gel containing GelRed nucleic acid stain (Gene Target Solutions, AUS) using Tris/Borate/EDTA (TBE) buffer. Amplicons presenting as single bands of the expected size were individually treated with ExoSAP-IT PCR Product Cleanup Reagent (Thermo Fisher Scientific, USA) and subjected to Sanger sequencing. Sequences were examined using Geneious Prime® 2021.2.2 and matched to data in the GenBank database using the BLASTn algorithm.
Statistical analyses
Prevalence data was analysed and displayed in the RStudio Team 2020 Integrated Development Environment for R (RStudio, PBC, Boston) and Microsoft Excel 2021; 95% confidence intervals (CIs) were calculated using the Wilson score interval via the open-source software Epitools (https://epitools.ausvet.com.au). Multiple logistic regression analyses were performed to infer associations with infection with gastrointestinal parasites, Toxocara, and hookworms with variables age, sex, BCS, lactation, health abnormalities, roaming behaviour and whether dogs had previously received an endoparasiticide, in Prism 9 (San Diego, CA, USA). A strategy of iterative backward-elimination was used to arrive at the final models. In each iteration, the explanatory variable that had the largest P value of those exceeding a threshold α = 0.2 was removed, and a new model was fitted. Associations between explanatory variables and the response variable were considered statistically significant if their P value was <0.05.
Results
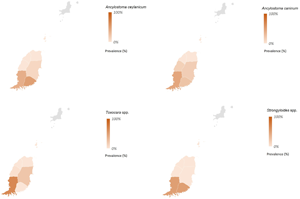
In total, 232 fecal samples were collected from dogs ranging in age from 2 weeks to 15 years; 93 and 139 dogs were <1 and >1 year/s of age. Microscopic examination revealed eggs of Ancylostoma spp. in 46.5% (108/232, 95% CI 40–52.9), Toxocara spp. in 9% (21/232, 95% CI 5.35–12.7) and T. vulpis in 5.2% (12/232, 95% CI 2.3–8) of fecal samples (Table 2, Fig. 1).

Figure 1. Prevalence of gastrointestinal parasites detected by microscopy and multiplex qPCR assays. Error bars indicate standard error mean.
Table 2. Number of animals <1 or >1 year of age positive for gastrointestinal parasites at microscopy (*) or qPCR (#) in 232 dogs from Grenada

Polyparasitism with STHs was recorded in 14% (19/137, 95% CI 9–21) of positive samples, of which 12 were positive for Ancylostoma spp. and Toxocara spp., 6 for Ancylostoma spp. and T. vulpis, and 1 for all 3 parasites. An additional 2 samples contained Ancylostoma spp. and Isospora spp. and 1 contained Ancylostoma spp. and Dipylidium caninum proglottids. Occasionally other parasites were found, including 3 separate samples positive only for D. caninum egg packets, Demodex spp. and Mammomonogamus spp..
In total, 211 of the 220 samples contained a sufficient amount of starting material for molecular analysis based on an assessment of the Ct values for individual samples using DNA-extraction control and were subjected to M-qPCR. Out of the 211 samples, 42.2% (89/211, 95% CI 35.5–48.8) were positive for at least 1 zoonotic parasite. Of these, 40.8% (86/211, 95% CI 34.1–47.3) were test-positive for Ancylostoma spp., of which 36% (76/211, 95% CI 29.5–42.9) were positive for A. caninum and 13.3% (28/211, 95% CI 9–18.6) for A. ceylanicum, 5.7% (12/211, 95% CI 2.97–8.81) were positive for T. canis, and 1% (2/211 95% CI 0–2.26) for Strongyloides spp. ((Zendejas-Heredia et al., Reference Zendejas-Heredia, Colella, Macpherson, Sylvester, Gasser, Macpherson and Traub2022; Table 2). Uncinaria stenocephala and A. braziliense positive samples were not detected by M-qPCR (Zendejas-Heredia et al., Reference Zendejas-Heredia, Colella, Macpherson, Sylvester, Gasser, Macpherson and Traub2022). Two samples were positive by M-qPCR for Strongyloides spp.; Sanger DNA sequencing showed for one of them a 99% nucleotide identity with publicly available sequences for S. stercoralis (GenBank Accession nos. KU724129, MN076383 and MK468672; haplotype B corresponding to the S. stercoralis dog cluster; cf. Barratt et al., Reference Barratt, Lane, Talundzic, Richins, Robertson, Formenti, Pritt, Verocai, Nascimento de Souza, Mato Soares, Traub, Buonfrate and Bradbury2019; the other sequences showed a 100% nucleotide identity to sequences of Strongyloides papillosus (AB923886 and LM525870). In addition, sequencing of the ITS-2 of Toxocara-positive microscopy samples showed 100% nucleotide identity with T. canis sequences available in GenBank (accession nos. OM87669-73, OK635791-92, MK728991-2 and MH044068-73). Sequences obtained for Strongyloides spp. and T. canis are available in GenBank under accession nos. OQ862314-15 and OQ909098 and OQ909099, respectively.
A multiple logistic regression model identified the variables BCS [OR 1.19 (95% CI: 1.03–3.58), P = 0.04] and the status of free-roaming dogs [OR 2.39 (95% CI: 1.38–4.16), P = 0.002] as significant predictors of test-positivity to at least 1 gastrointestinal parasite (Table 2). Evidence of an association between free-roaming dogs [OR 1.9 (95% CI: 1.10–3.31), P = 0.004] and hookworm infection and BCS [OR 0.08 (95% CI: 0.004–0.42), P = 0.017] and Toxocara spp. infection was identified (Table 3).
Table 3. Parameter estimates and odds ratios (95% profile likelihood) for positivity to at least 1 gastrointestinal (GI) parasites and hookworms in 232 dogs from Grenada

Discussion
Several zoonotic nematodes were reported as being endemic in the canine population of Grenada and both canine roaming behaviour and health status were identified as predictors for parasitism by multiple gastrointestinal nematodes. Using molecular tools, S. stercoralis and S. papillosus DNA were detected in individual dogs. The finding of S. papillosus is likely pseudoparasitism due to coprophagy of ruminant feces by dogs. The genetic relationship of S. stercoralis between dogs and humans remains unclear; haplotype A is considered zoonotic, whereas haplotype B has only been isolated from dogs (Ko et al., Reference Ko, Suzuki, Canales-Ramos, Aung, Htike, Yoshida, Montes, Morishita, Gotuzzo, Maruyama and Nagayasu2020). As Strongyloides DNA was detected in only 2 fecal samples here, it is likely that Strongyloides spp. may not be significant contributors to canine ill health in Grenada. Microscopic examination did not reveal larvae in any of the 232 samples, which is not surprising, given the limited fecundity of adult Strongyloides spp. and/or the tendency of larvae to hatch in the gut which are not readily detected using a conventional flotation-microscopy method. Each year, a number of human cases of hyperinfection syndrome of strongyloidiasis are reported by clinicians at the Grenada General Hospital. These cases may occur in patients with HTLV-1, as Grenada has the second highest known prevalence of this infection with immunocompromising virus in the world (Willems et al., Reference Willems, Hasegawa, Accolla, Bangham, Bazarbachi, Bertazzoni, Carneiro-Proietti, Cheng, Chieco-bianchi, Ciminale, Coehlo-Dos-Reis, Esparza, Gallo, Gessain, Gotuzzo, Hall, Harford, Hermine, Jacobson, Macchi, Macpherson, Maheiux, Matsuoka, Murphy, Peloponese, Simon, Tagaya, Taylor, Watanabe and Yamano2017). Therefore, future studies – using a larger sample size – will be required to determine the prevalence of S. stercoralis in dogs in Grenada and to assess whether the zoonotic haplotype is present in this country.
Ancylostoma caninum was the predominant hookworm species found in Grenada (Zendejas-Heredia et al., Reference Zendejas-Heredia, Colella, Macpherson, Sylvester, Gasser, Macpherson and Traub2022). The absence of A. braziliense, which is the most common cause of long lasting, serpiginous CLM, was surprising and needs further investigation, as the latter species appears to be rarer than the other hookworm species. This finding is consistent with the observation by local physicians that CLM is not commonly seen, despite a high infection rate of hookworm in dogs that roam frequently visited areas, such as the main tourist beach in Grenada (Schwartz et al., Reference Schwartz, Bidaisee, Fields, Macpherson and Macpherson2022; Neill, personal communication, Dec. 2009). The new molecular evidence suggests that A. braziliense may not be as widespread as previously thought, although additional studies of cats should be conducted, as felines can also serve as hosts (Bowman et al., Reference Bowman, Montgomery, Zajac, Eberhard and Kazacos2010).
Of the Toxocara species, T. canis was confirmed as present in this population using PCR techniques and sequencing. While T. canis antibodies have been recently serologically detected in people in Grenada (Ziegler, Reference Ziegler2022, unpublished results), no official reports of clinically significant infection have been documented (Schwartz et al., Reference Schwartz, Bidaisee, Fields, Macpherson and Macpherson2022). Implementation of prevention programmes for zoonotic STHs including Toxocara spp. in the canine population would likely help keep human clinical infection rates low.
Here, 14% of dogs positive for at least 1 STH showed polyparasitism. Roaming behaviour and BCS were determined to be significant predictors of test-positivity for STHs in dogs. Roaming dogs likely encounter numerous pathogens as they frequent many different properties and interact with numerous environments, other animals and people. Body condition is a parameter regularly used to assess health status in dogs by estimating fat deposits around specific parts of the body and is often recorded on the lower or skinnier end in dogs with parasitic infections (Fung et al., Reference Fung, Calzada, Saldana, Santamaria, Pineda, Gonzalez, Chaves, Garner and Gottdenker2014). In this study, dogs with a low BCS were more likely to be infected with at least 1 of the gastrointestinal parasites investigated than dogs that were of optimal weight. A higher BCS was associated with Toxocara spp. infection, which may be attributable to the subjective physical assessment of pot-bellied puppies and pregnant dogs found to be infected.
Appropriate timing of anthelmintic treatment, especially targeting the prevention of transplacental/transmammary transmission for Toxocara and hookworm, would be an effective means of reducing parasitic transmission but requires funds to support veterinary care which is often limited in low- and middle-income countries. Such a strategy would reduce the imperative for owners to have their puppies treated within the first 2 weeks postpartum to prevent egg shedding in this highly infected population (Gates and Nolan, Reference Gates and Nolan2009). Emphasizing the importance of public education to reduce roaming behaviour in dogs, remove and dispose of canine feces and wash hands regularly are also important means of controlling transmission (Macpherson et al., Reference Macpherson, Pinckney, Sylvester, Bidaisee and Macpherson2022; Massetti et al., Reference Massetti, Wiethoelter, McDonagh, Rae, Marwedel, Beugnet, Colella and Traub2022; Walker et al., Reference Walker, Lambert, Neves, Worsley, Traub and Collela2023). The presence of A. ceylanicum requires that further behavioural changes be adopted as well, such as wearing shoes outdoors and proper disposal of human and animal feces (Baker et al., Reference Baker, Trinies, Bronzan, Dorkenoo, Garn, Sognikin and Freeman2018; Nery et al., Reference Nery, Pickering, Abate, Asmare, Barrett, Benjamin-Chung, Bundy, Clasen, Clements, Colford, Ercumen, Crowley, Cumming, Freeman, Haque, Mengistu, Oswald, Pullan, Oliveira, Owen, Walson, Youya and Brooker2019; Colella et al., Reference Colella, Khieu, Worsley, Senevirathna, Muth, Huy, Odermatt and raub2021; Walker et al., Reference Walker, Lambert, Neves, Worsley, Traub and Collela2023). Further studies of Strongyloides spp. in children and immunocompromised individuals are warranted, as well as studies of A. ceylanicum in people >12 years of age, as this demographic comprises >80% of hookworm-infected individuals in the tropics (Nutman, Reference Nutman2017; Colella et al., Reference Colella, Khieu, Worsley, Senevirathna, Muth, Huy, Odermatt and raub2021).
In conclusion, the livelihoods of dogs in Grenada provide ideal conditions for parasite transmission and may possess the potential for zoonotic spread. This study stimulates future, larger-scale investigations of zoonotic parasitic helminths in animals and humans in Grenada and other parts of the West Indies using molecular diagnostic tools in order to elucidate their public health impact.
Data availability
Sequence data are available from GenBank, under accession numbers KU724129, MN076383, MK4686, AB923886, and LM525870.
Author contributions
C. N. L. M., V. C., R. B. G. and R. T. were responsible for study design and supervision of the study. M. L. A. M. and W. S. collected samples in the field and performed microscopy in the laboratory. P. Z. H. and V. C. conducted molecular and statistical analysis. R. B. G. and R. T. contributed to the interpretation of this data. M. L. A. M. and C. N. L. M. wrote the first draft of the paper. All authors contributed to the revision of the paper and approved the final draft.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
This project was approved by the Institutional Animal Care and Use Committee (IACUC) of the St. George's University, Grenada. IACUC approval number: IACUC-21001-R.