Lutein is a xanthophyll carotenoid found particularly in dark-green leafy vegetables. It is widely distributed in tissues and is one of the principal carotenoids in the eye lens and macular region of the retina, being dominant in the more external portions of the retina(Reference Landrum and Bone1, Reference Krinsky2). Owing to its absorbent properties in relation to luminous radiation and antioxidant capabilities, this xanthophyll is an interesting compound for the health of the eye(Reference Landrum, Bone, Joa, Kilburn, Moore and Sprague3–Reference Conn, Schalch and Truscott5). The action of lutein appears to be important in this context thanks to its considerable filtering capacity as regards light(Reference Junghans, Sies and Stahl6) and its antioxidant action.
There are still few studies that have highlighted the possible role of lutein in the long-term prevention of morbidity and disability in neonates. The oxidative stress to which an infant is subjected at birth has been considered a risk factor in the aetiology of damage to the visual apparatus and more besides. As for age-related macular degeneration in the elderly, where free radicals are today considered an important risk factor, even in neonates, antioxidants could play a protective role. Lutein, especially, may have an action countering the pro-oxidant effects of light by absorbing radiation and neutralizing singlet oxygen and free radicals that are produced in the metabolic processes of retinal ischaemic tissue(Reference Jewell, Northrop-Clewes, Tubman and Thurnham7).
Among its components, breast milk has a large amount of antioxidants, including lutein, that may play an essential role for newborn eye health, given that this carotenoid seems to have specific transporters widely represented at the level of the placenta, the mammary gland and the ocular tissue(Reference Krinsky, Mayne and Sies8). This carotenoid may protect the neonate’s retina from light-induced oxidative damage that it may encounter in the first months of life(9, Reference Hylander, Strobino, Pezzullo and Dhanireddy10); indeed, in this period, lutein in breast milk may help to accommodate the newborn’s transition from the darkness experienced in utero.
Levels of lutein in human milk are two to three times higher than those of β-carotene(Reference Hylander, Strobino, Pezzullo and Dhanireddy10); thus maternal milk is the main dietary source of lutein for infants until weaning occurs. Investigation of maternal milk lutein concentration during the first month postpartum may provide information useful to suggest dietary recommendations during both pregnancy and lactation, in order to satisfy both infant and maternal requirements. The few studies that have been conduced in lactating women report great variability of dietary lutein intakes among countries, ranging from 2108 to 4929μg/d(Reference Gossage, Deyhim, Yamini, Douglass and Moser-Veillon11, Reference Canfield, Giuliano, Neilson, Yap, Graver, Cui and Blashill12).
The present study aimed to estimate the lutein concentration in human milk during early lactation in a group of puerperae and its relationship with dietary lutein intake measured through the administration of a short FFQ(Reference Cena, Roggi and Turconi13).
Materials and methods
Sample
Twenty-one pregnant women were recruited on a voluntary basis, during their last trimester, at the Obstetric Unit of Pavia University in northern Italy.
Written informed consent was obtained from all participants prior to their inclusion in the study, which was performed in accordance with the ethical standards laid down in the appropriate version of the 1994 Declaration of Helsinki and approved by the University of Pavia’s Faculty of Medicine Ethical Committee.
Subjects, in good health and nutritional state as assessed by anthropometric measurements and laboratory tests, were eligible to join the study if they did not smoke, had not taken prenatal supplements containing lutein, did not consume alcohol and had no unusual dietary habits due to spontaneous intake restrictions, vegetarianism, known food allergies, etc.
Six women dropped out during the trial period.
Study design
In the present cross-sectional study, a previously validated FFQ(Reference Cena, Roggi and Turconi13) was administered twice: on day 3 (T0) and day 30 (T1) postpartum, in order to estimate dietary lutein intake; meanwhile, two breast milk samples were collected. In addition, maternal plasma samples were obtained on the third day of puerperium (T0).
Intake assessment
A brief FFQ was previously developed(Reference Cena, Roggi and Turconi13) providing a list of fruit and vegetables typically consumed by the Italian adult population according both to the Mediterranean diet(Reference Turrini, Saba, Perrone, Cialfa and D’Amicis14) and the FFQ used in the European Prospective Investigation into Cancer and Nutrition(Reference Pasanisi, Berrino, Bellati, Sieri and Krogh15, Reference Pala, Berrino, Vineis, Palli, Celentano, Tumino and Krogh16), ending up with a final list of thirty items (dark-green leafy vegetables as well as green peas, summer squash, broccoli, lettuce and corn; fruits such as tangerines, peaches and oranges). The FFQ was then validated by comparing the results obtained with a 7 d dietary record as a reference standard(Reference Cena, Roggi and Turconi13).
Data collection
We chose to administer the questionnaire by interview since some problems may arise with self-administration; for example, answers may be incomplete as some respondents will only complete the questionnaire for items they usually eat.
Frequency of usual food consumption was investigated by inviting the respondents to report their consumption as ‘never consumed’ or in units of their choice such as ‘number of occasions’ per day, week or month, rather than being restricted to specific frequency ranges with a reference period of ‘over the past month’ as already reported by other authors(Reference Neuhouser, Rock, Eldridge, Kristal, Patterson, Cooper, Neumark-Sztainer, Cheskin and Thornquist17, Reference Rock, Thornquist, Neuhouser, Kristal, Neumark-Sztainer, Cooper, Patterson and Cheskin18). Usual dietary lutein intake was assessed with this quantitative thirty-item FFQ.
Summary questions aimed at obtaining overall information on the number of servings of fruit and vegetables per day, as well as questions for cross-checking the consistency of answers, were asked during the interviews.
To quantify the food portions sizes consumed, a photographic food atlas was used(Reference Turconi and Roggi19, Reference Turconi, Guarcello, Gigli Berzolari, Carolei, Bazzano and Roggi20).
Patients reported that the FFQ was easy to understand, clear, short and not demanding. Furthermore, the use of the colour photographic food atlas helped to hold the attention of the subjects being interviewed.
All interviews were performed at the Obstetric Unit of Pavia University by a highly trained dietitian who had received 3 h of instruction. Completing the questionnaire took on average 10 min. The questionnaire was administered twice, when the milk samples were being taken.
Collection of milk and blood samples
The breast milk and blood samples were collected in the period immediately following delivery: on day 3 postpartum (T0). Fifteen subjects provided a second milk sample on day 30 (T1) postpartum.
The blood samples were taken after an overnight fast from the ulnar vein, in a quantity of 10 ml. The samples were transferred to test tubes containing 0·1 % EDTA, subsequently conserved in ice at 4°C and transported on dry ice to the analysis laboratory within 4 h. The blood was then centrifuged for 20 min at 4°C and 3000g in order to separate the plasma. Two millilitres of plasma from each sample were then analysed.
Milk samples were taken using the breast pump available at the university obstetric clinic. The milk was obtained from one breast in an amount equal to about 5–6 ml. After collection, the milk samples were protected from light sources and also transported on dry ice directly to the laboratory, where they were quantified and conserved at −80°C. The analyses of these samples were performed within a month of collection, a period during which the analytes remain stable according to the literature(Reference Comstock, Alberg and Helzlsouer21).
Lutein analysis in milk and plasma samples
All analyses were conducted under subdued lighting to avoid degradation of lutein. Samples were allowed to thaw and come to room temperature on a shaker.
Lutein (standard) and β-apo-8′-carotenal (internal standard) were obtained from Kemin Industries and Fluka, respectively.
Milk (2 ml) was saponified by adding 0·5 ml of potassium hydroxide (40 % in methanol) and 0·1 ml of β-apo-8′-carotenal (internal standard, 20 μmol/l) in methanol. The sample was placed in a water bath at 45°C for 30 min and then lutein was extracted from the saponified matrix using 1·5 ml of hexane (containing 0·01 % butylated hydroxytoluene (BHT) w/v) for three times. Combined extracts were dried under nitrogen and then reconstituted in 0·5 ml of isopropanol–hexane (10:90 v/v); 50 μl were injected onto the column(Reference Jewell, Mayes, Tubman, Northrop-Clewes and Thurnham22).
Plasma (0·5 ml) was mixed with ethanol (1 ml) containing β-apo-8′-carotenal (internal standard, 3 μmol/l) and BHT (0·01 % w/v), ethyl acetate (1 ml) and n-hexane (1 ml). The mixture was vortexed and then centrifuged (1 min at 1200g). The supernatant solution was separated, then the pellet was vortexed and centrifuged as before with hexane (1 ml) twice. Water (0·5 ml) was added to the pooled supernatant solution, which was vortexed and centrifuged as before. The hexane phase was carefully pipetted into a glass tube, evaporated to dryness under a stream of nitrogen and then reconstituted in 0·5 ml isopropanol–hexane (10:90 v/v); 50 μl were injected onto the column(Reference Barua23).
Samples were analysed with a Thermo Separation Product HPLC, equipped with a P200 pump, an AS3000 autosampler and a Spectra Focus scanning detector, set to 450 nm. A straight phase gradient analysis was carried out, using a Merck Superspher Si 60 column (4 μm, 4 mm × 250 mm); the mobile phase was a mixture of 2-propanol (A) and hexane (B), with a gradient program as follows:
1. Isocratic 0:100 (A:B) for 2·5 min;
2. Linear gradient from 0:100 to 18:82 (A:B) from 2·5 to 18 min;
3. Isocratic 18:82 (A:B) from 18 to 24 min;
4. A 2-min gradient back to 0:100 (A:B).
Flow rate was 1 ml/min and retention time of lutein was about 20 min. In addition to the internal standard, spiking experiments were performed using breast milk, in order to ensure adequate recovery of lutein. The average recovery for five replicate analyses was 85·4 % and the range was 82·1–88·8 %. The limit of detection in milk and plasma was 0·025 and 0·100 μmol/l, respectively.
Statistical analysis
The FFQ were analysed and lutein intakes were calculated using the US Department of Agriculture–National Cancer Institute Carotenoids Database(Reference Holden, Eldridge, Beecher, Buzzard, Bhagwat, Davis, Douglass, Gebhardt, Haytowitz and Schakel24). Dietary intakes of lutein, estimated on the basis of the FFQ, were analysed by calculating the means and standard deviations.
There were two repeated measures for milk values as well as two repeated estimations for dietary lutein intakes (day 3 and day 30 postpartum), while plasma analyses included only one measure (day 3 postpartum). Comparison between FFQ administered at T0 and T1 on fifteen women to estimate intake of lutein, and likewise comparison of milk lutein concentration (at T0 and T1 on the same sample), was analysed with the paired t test. A normal probability plot was used to assess whether or not the data sets were normally distributed.
Pearson’s correlation coefficient was used in order to determine the association between dietary lutein intake and lutein concentration in milk and plasma, respectively. The same coefficient was also used to calculate the correlation between breast milk and plasma lutein concentrations at T0.
Data were analysed with the SPSS statistical software package version 13 (SPSS Inc., Chicago, IL, USA). All results are reported as means and standard deviations.
Results
Out of twenty-one women enrolled, only fifteen completed the study. Of the six subjects who dropped out, two changed their mind and decided to go elsewhere for their 1-month postpartum check-up, one started smoking and three decided to suspend breast-feeding for formula milk feeding.
Most of the subjects were middle-class and well educated, age range 24–42 years (Table 1). Approximately half of the subjects had taken prenatal dietary supplements regularly, which however did not contain lutein.
Table 1 Characteristics of the sample: lactating women (n 21) aged 24–42 years, Pavia, Italy

At T0, estimated dietary intake of lutein obtained from the FFQ was 1242 (sd 113) μg/d; breast milk lutein and plasma lutein concentrations in the whole sample (n 21) were 0·26 (sd 0·19) and 0·69 (sd 0·49) μmol/l, respectively.
Both breast milk and plasma lutein concentrations were significantly correlated with dietary lutein intake (r = 0·86, P = 0·0001 and r = 0·94, P = 0·0001, respectively). Furthermore, there was a clear significant correlation between milk and plasma lutein concentrations (r = 0·87, P = 0·0001; Fig. 1).

Fig. 1 Correlations between estimated dietary lutein intake and plasma and breast milk lutein concentrations at T0 (day 3 postpartum) among lactating women (n 21) aged 24–42 years, Pavia, Italy. All correlations were statistically significant: (a) r = 0·86, P = 0·0001; (b) r = 0·94, P = 0·0001; (c) r = 0·87, P = 0·0001
In addition, breast milk lutein concentrations were significantly correlated with the number of portions of fruit (2·10 (sd 1·06)) and vegetables (1·72 (sd 1·20)) consumed daily (r = 0·72, P = 0·01 and r = 0·68, P = 0·001, respectively).
The fifteen subjects who ended the study provided another milk sample on day 30 postpartum (T1). On that occasion they also completed another FFQ.
At T0, estimated dietary lutein intake obtained from the FFQ of these fifteen subjects was 1209 (sd 157) μg/d; breast milk lutein and plasma lutein concentrations were 0·28 (sd 0·22) and 0·67 (sd 0·42) μmol/l, respectively (Table 2). There were no significant differences among these variables compared with those of the whole sample.
Table 2 Lutein intake, mean plasma and breast milk lutein concentration in lactating women, Pavia, Italy
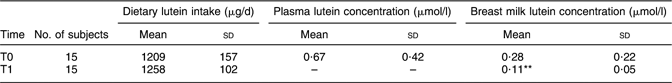
T0, day 3 postpartum; T1, day 30 postpartum.
Mean value was significantly different from that at T0 (Student’s t test): **P < 0·01.
At T1, estimated dietary lutein intake was 1258 (sd 102) μg/d, revealing no significant differences with either that estimated in the same sample (n 15; 1209 (sd 157) μg/d, P = 0·39) or that estimated in the whole sample (n 21; 1242 (sd 113) μg/d, P = 0·27) at T0.
Mature milk lutein concentration at T1 was 0·11 (sd 0·05) μmol/l (Table 2). Plasma samples at T1 were not obtained. All the data were normally distributed.
Compared with the value reported at T0, mature milk lutein concentration decreased significantly over time (P < 0·01), while there were no statistically significant changes in intake of this carotenoid over time. Breast milk lutein concentration at T1 maintained a fairly high correlation with estimated dietary lutein intake (r = 0·82, P = 0·0001; Fig. 2).

Fig. 2 Correlation between estimated dietary lutein intake and breast milk lutein concentration at T1 (day 30 postpartum) among lactating women (n 15) aged 24–42 years, Pavia, Italy. Correlation was statistically significant: r = 0·82, P = 0·0001
Discussion
The present study aimed to estimate lutein concentration in human milk during early lactation and its relationship with dietary intake of this carotenoid.
Our population is small but there are many difficulties inherent to the enrolment of pregnant/postpartum women. On the other hand, we are confident that our results may add knowledge on research aimed at studying the protective role of these compounds in well-nourished pregnant women and their newborns.
The significant correlations observed at T0 in the present study indicate that dietary intakes may provide valuable predictive information on maternal milk and plasma lutein concentrations. In addition, correlations between breast milk lutein concentration and daily servings of fruit and vegetables appear to be sufficient as an indicator of dietary lutein intake, suggesting that eating selected fruit and vegetables regularly leads to a progressive increase in maternal milk lutein concentration.
Dietary lutein intake as well as milk and plasma lutein values measured in the present research are similar to those of other studies, although authors report that both breast milk and plasma lutein concentrations show considerable inter-individual variability(Reference Macias and Schweigert25, Reference Meneses and Trugo26).
We observed that dietary lutein intake was correlated both with breast milk (r = 0·86, P = 0·0001) and plasma lutein concentrations (r = 0·94, P = 0·0001), as already reported by other authors(Reference Gossage, Deyhim, Yamini, Douglass and Moser-Veillon11, Reference Canfield, Clandinin and Davies27). The strong correlation between dietary lutein intake and plasma lutein concentration is in agreement with our previous results(Reference Cena, Roggi and Turconi13), as well as other data(Reference Bone, Landrum, Dixon, Chen and Llerena28). From further analyses a significant correlation also emerged between maternal milk and plasma lutein concentrations (r = 0·87, P = 0·0001). A similarly high correlation has already been reported by other authors(Reference Lietz, Mulokozi, Henry and Tomkins29, Reference de Azeredo and Trugo30), although weaker correlations have also been described in the literature(Reference Gossage, Deyhim, Yamini, Douglass and Moser-Veillon11). Breast milk lutein concentration in our study was equal to approximately 40 % of the plasma value, data that are supported by other studies(Reference Canfield, Giuliano, Neilson, Yap, Graver, Cui and Blashill12, Reference Canfield, Giuliano, Neilson, Blashil, Graver and Yap31).
The present study also sought to compare the difference in milk lutein concentration at 1 month postpartum, to observe changes in concentration of this carotenoid over time as well as changes in its relationship with dietary lutein intake.
Comparing lutein intakes assessed at T0 and T1, day 3 and 30 postpartum respectively, we determined that there were no changes in intake of this nutrient over time, assuming no significant variations in maternal diet composition. Daily lutein intake in our sample is similar to that obtained by other authors(Reference Cena, Roggi and Turconi13, Reference Rock, Thornquist, Neuhouser, Kristal, Neumark-Sztainer, Cooper, Patterson and Cheskin18, Reference Curran-Celentano, Hammond, Ciulla, Cooper, Pratt and Danis32, Reference Hammond, Ciulla and Snodderly33).
According to the literature(Reference Canfield, Giuliano, Neilson, Yap, Graver, Cui and Blashill12, Reference Canfield, Giuliano, Neilson, Blashil, Graver and Yap31), among lactating mothers in good health, breast milk carotenoid concentrations vary widely. Previous studies have identified women’s dietary habits as the variable with the greatest influence on plasma carotenoid concentrations(Reference Broekmans, Klöpping-Ketelaars, Schuurman, Verhagen, van den Berg, Kok and van Poppel34, Reference Tucker, Chen, Vogel, Wilson, Schaefer and Lammi Keefe35); the same relationship seems to exist between dietary intakes and breast milk concentrations.
In our study, mature milk lutein concentration was significantly decreased (P < 0·01) on day 30 postpartum, suggesting that lutein in breast milk appears to almost stabilize at 1 month postpartum. Our results are similar to those of many other studies, which have highlighted the fact that carotenoid concentrations (lutein, zeaxanthin, β-cryptoxanthin, lycopene, α- and β-carotene, retinol) in breast milk decrease over time, showing higher concentrations in colostrum compared with mature milk, enhancing however the importance of lutein compared with the others(Reference Gossage, Deyhim, Yamini, Douglass and Moser-Veillon11, Reference Jewell, Mayes, Tubman, Northrop-Clewes and Thurnham22, Reference Macias and Schweigert25, Reference Sommerburg, Siems, Hurst, Lewis, Kliger and van Kuijk36). It has been reported that lutein represents approximately 25 % of the milk carotenoid composition on day 3 postpartum, but approaches 50 % on day 30 postpartum (mature milk)(Reference Gossage, Deyhim, Yamini, Douglass and Moser-Veillon11). Other studies report these milk lutein concentration changes along with a decrease of plasma lutein concentration(Reference Gossage, Deyhim, Moser-Veillon, Douglas and Kramer37). In our study, it would have been interesting therefore also to evaluate the mothers’ plasma lutein concentrations at day 30 postpartum; the lack of such data highlights a limitation of our study.
Since dietary lutein intake did not change in our sample during the 1-month observation it may be suggested that the flow of lutein through the mammary gland could be altered in the postpartum period, implying that some event or set of events associated with parturition, initiation of lactation or both may affect lutein absorption, metabolism or both. These events might include postpartum normalization of lipoprotein concentration or changes in adiposity. It is difficult to speculate about the nature of these events on the basis of the limited data we collected.
The exact lutein secretion mechanism remains to be clarified, and may be different during colostrogenesis and the phases following lactogenesis. It could be linked to selective absorption of lipids through specific lipoprotein fractions, as indicated from the distribution pattern of carotenoids among these vehicles, or to particular mechanisms across membrane transporters and specific channels(Reference Macias and Schweigert25, Reference Lietz, Mulokozi, Henry and Tomkins29, Reference Schweigert, Bathe, Chen, Büscher and Dudenhausen38).
Conclusions
Further research is needed to determine whether the observed postpartum decrease in milk lutein is simply normalization and to investigate the cause and potential role of breast milk lutein concentration changes during early lactation.
Even though milk lutein concentration decreases during early lactation, it remains significantly correlated to daily lutein intake. Therefore, while awaiting further research, dietary recommendations advising intake of fresh fruit and vegetables rich in lutein, throughout the whole duration of pregnancy and lactation, are extremely useful.
Acknowledgements
The present research was funded by the IRCCS Policlinico S. Matteo, Pavia, Italy. There are no financial or other contractual agreements that might cause conflicts of interest. Contributions of the authors are as follows: H.C. and G.T., conception and design of the study, generation and interpretation of data, revision of the manuscript; A.M.C., conception and design of the study, generation and collection of data; A.P., laboratory analysis; C.R., conception and design of the study. The authors state that: none of the material in the present manuscript is included in another manuscript nor has been or is currently under consideration for publication elsewhere; no portion of the manuscript has been published or posted on the Internet; ethical guidelines were followed by the authors in performing the study, which has been approved by the institutional human ethics committee; and each author has participated actively in the work as reported above and has given substantial contribution. Each author has read and approved the final submitted manuscript. The IRCCS Policlinico S. Matteo, Pavia, Italy is thanked for funding the research.








