Implications
On Mediterranean goat farms, the use of the male effect in spring is a common practice. Spring is when normal male reproductive activity is diminished, but it can be induced by subjecting them to three months of long days. Mating female goats aged 7 to 10 months in spring is difficult; apart from the season being unfavourable, they have not yet entered puberty. The present results show that does aged 7 or 10 months enter puberty sooner, and thus return better reproductive results, if exposed to photostimulated males than non-treated males.
Introduction
Puberty in female goats may be defined as the time at which oestrus is first detected by a male, followed by cyclical ovarian activity (Greyling, Reference Greyling2000). In seasonal breeders such as sheep and goats, puberty is reached during the breeding season. The interval between weaning and the onset of reproductive activity is thus an unproductive period for stock-raisers, and reducing its length, that is, allowing does to be mated at an earlier age, would likely increase their lifetime productive performance and reduce flock production costs.
A number of environmental factors influence the onset of puberty in does, including the photoperiod and season of birth (kids experience different photoperiodic stimuli depending on the time of year when they are born; age at puberty therefore differs according to the season of birth), body weight (BW), body condition (BC), and the male effect (Delgadillo et al., Reference Delgadillo, De Santiago-Miramontes and Carrillo2007; Zarazaga et al., Reference Zarazaga, Guzmán, Domínguez, Pérez, Prieto and Sánchez2009; Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015). Summer/autumn-born does cannot be mated during the ensuing breeding season since they will either not have reached puberty, or barely reached it with just the minimum BW required (Kenyon et al., Reference Kenyon, Viñoles and Morris2012). Such does can only be mated in the following year when they are 1 or 1.5 years old (Papachristoforou et al., Reference Papachristoforou, Koumas and Photiou2000; Delgadillo et al., Reference Delgadillo, De Santiago-Miramontes and Carrillo2007; Zarazaga et al., Reference Zarazaga, Guzmán, Domínguez, Pérez, Prieto and Sánchez2009).
Numerous studies have reported a close association between body growth, nutritional status and the timing of puberty (Frisch and Revelle, Reference Frisch and Revelle1970; Frisch et al., Reference Frisch, Hegsted and Yoshinaga1977; Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015). Several lines of evidence suggest that, in mammals, a critical BW (Frisch and Revelle, Reference Frisch and Revelle1970) or critical BC (Frisch et al., Reference Frisch, Hegsted and Yoshinaga1977) must be reached if puberty is to be attained at the expected time. Gallego-Calvo et al. (Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015) suggest that upon reaching this critical BW, the BC becomes a determining factor in the onset of puberty.
The induction of the male effect in adult does is a common practice in extensive and semi-extensive goat-raising systems in Mediterranean countries. However, the degree of sexual activity displayed by males in spring – when the male effect is required, but when they show the least sexual interest – could influence the response of females (Flores et al., Reference Flores, Véliz, Pérez-Villanueva, Martínez de la Escalera, Chemineau, Poindron, Malpaux and Delgadillo2000; Delgadillo et al., Reference Delgadillo, Carrillo, Morán, Duarte, Chemineau, Malpaux and Mora2001 and Reference Delgadillo, Flores, Duarte, Vielma, Hernández, Bedos, Fitz-Rodríguez, Fernández, López-Sebastián, Gómez-Brunet, Santiago-Moreno, Zarazaga, Keller and Chemineau2014). In Mediterranean and subtropical latitudes, bucks rendered sexually active by exposure to long days appear to induce the sexual activity of seasonally anoestrous does better than bucks kept under the natural photoperiod (Zarazaga et al., Reference Zarazaga, Gatica, Celi, Guzmán and Malpaux2010 and Reference Zarazaga, Gatica, Gallego-Calvo and Guzmán2018; Chasles et al., Reference Chasles, Chesneau, Moussu, Delgadillo, Chemineau and Keller2016). This raises the question of whether photostimulated males are also able to stimulate puberty in young females. In sheep, Kenyon et al. (Reference Kenyon, Viñoles and Morris2012) demonstrated that when 8-month-old ewe lambs were exposed to teaser rams, a greater proportion displayed oestrous. Moreover, Chasles et al. (Reference Chasles, Chesneau, Moussu, Delgadillo, Chemineau and Keller2017) and Espinoza-Flores et al. (Reference Espinoza-Flores, Hernández, Andrade-Esparza, Ramírez, Zarazaga, Chemineau, Keller and Delgadillo2018) recently showed that, in goats, exposure to sexually active males advances puberty in females over that seen in those isolated from males.
On the basis of these results, it was hypothesized that, at Mediterranean latitudes, the male effect induced by photostimulated bucks might induce puberty in young does, perhaps independent of doe age.
Material and methods
Study conditions
All procedures were performed by trained personnel in strict accordance with Spanish guidelines for the protection of experimental animals (RD 53/2013), and in agreement with European Union Directive 86/609. The study was conducted at the University of Huelva experimental farm (latitude 37° 20’N and longitude 6° 54’W), which meets the requirements of the European Community Commission for Scientific Procedure Establishments (2010/63). Methods were similar to those used in similar works of our research group (Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015; Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Guzmán and Zarazaga2018).
Experimental design
The experiment was a 2×2 factorial design, consisting of a doe age and buck photoperiod treatments. The does used (n=41) were divided into two age groups before exposure to the males (introduction): (1) 7-month-old group (n=19; age 221±0.42 days at introduction (born on 1 September±0.42 days); BW 23.5±0.40 kg; BC score 2.95±0.04), and (2) 10-month-old group (n=22; age at introduction 292±0.92 days (born on 22 June±0.92 days); BW 28.9±0.40 kg; BC score 3.08±0.04). These groups were then subdivided, exposing half to photostimulated bucks (PHOTO bucks) (n=9 for the 7-month olds and 11 for the 10-month olds), and half to unstimulated bucks (CONTROL bucks) (n=10 for the 7-month olds and 11 for the 10-month olds).
The animals of each experimental age group were kept together in separate pens until the introduction of the males when the four subgroups were established. Feeding was adjusted weekly in relation to the BW to ensure a weight gain of 75 g per day, in accordance with the Institut National de la Recherche Agronomique standards (Morand-Fehr and Sauvant, Reference Morand-Fehr and Sauvant1988). All animals were fed concentrate, a commercial mixture of maize (26.3%), beans (20%), oats (14.1%), cotton-seed (13.7%), peas (13.4%), lupin (7.3%), barley (0.2%), wheat (0.2%), sunflower seeds (0.2%), a mineral–vitamin complement (4.6%) and barley straw. The nutritional values of the concentrate was 0.93 milk fodder units (UFL) and 76 g digestible protein per kilogram of dry matter (DM), while that of the barley straw was 0.37 UFL and 25 g of digestible protein per kilogram of DM. Concentrate was offered once a day and distributed individually; barley straw was administered ad libitum. All animals had free access to water and mineral blocks containing trace elements and vitamins.
Preparation of the males
At the latitude where the work was performed, male sexual rest lasts from January to February, to June to July (Zarazaga et al., Reference Zarazaga, Guzmán, Domínguez, Pérez, Prieto and Sánchez2009). Four young entire males (1.5 years old) were exposed to 3 months of long days (16 h of light per day) (PHOTO bucks) from 31 October, and thereafter to natural photoperiodic conditions. These long days were achieved by providing extra light beyond the natural daylength (at least 300 lux at the animals’ eye level, from 0600 to 0800 h and from 1900 to 2200 h), employing fluorescent lights. The PHOTO males were located in an open barn with access to an uncovered area. During the extra light periods and night the access to the uncovered area was closed. This treatment stimulates testosterone secretion and sexual behaviour in bucks during March and April, that is, the natural sexual rest period when control males are sexually inactive (Zarazaga et al., Reference Zarazaga, Gatica, Celi, Guzmán and Malpaux2010). Another four young entire males (1.5 years old) completely isolated from the PHOTO bucks (located in another open barn), were exposed to natural photoperiodic conditions during the whole experimental period (CONTROL bucks).
Determination of buck plasma testosterone concentrations
Blood was sampled weekly at 0900 h, from 31 October until 12 May. Plasma testosterone concentrations were determined using a commercial enzyme-linked immunoassay (ELISA) kit (Demeditec Diagnostics, Kiel-Wellsee, Germany). The sensitivity of the assay was 0.1 ng/ml. Intra- and inter-assay coefficients of variation for sample pools of 0.2 and 6.0 ng/ml were 1.6%, 2.3%, and 4.5% and 2.3%, respectively.
One week before introducing the bucks to the does, the sexual behaviour of the bucks was assessed by observing genital sniffing, nudging and mounting attempts over a 5 min period when the bucks were exposed to females (not those in experimental groups) in oestrus. The bucks within each photoperiod treatment group showed similar sexual behaviour; they were therefore randomly allocated to the appropriate doe subgroups.
The male effect
On 10 April, 69 days after the end of the photoperiod treatment, the males, equipped with marking harnesses, were introduced to the does (two per subgroup) and maintained with them for the following 32 days (until 12 May) to induce the male effect. All four experimental groups was located in open barns completely isolated from the other ones.
Variables recorded for the does
BW and body condition (BC)
The BW and BC of all the does were recorded weekly. The BC was scored by lumbar palpation (always by the same handler) based on a scale of 0=emaciated to 5=very fat, with increments of 0.25 (Hervieu et al., Reference Hervieu, Morand-Fehr, Schmidely, Fedele and Delfa1991).
Detection of oestrous behaviour, ovulation and ovulation rate
Oestrous activity was recorded every day by direct visual observation of the marks left by male-worn marking harnesses (Walkden-Brown et al., Reference Walkden-Brown, Restall and Henniawati1993). The interval between introduction and first oestrous behaviour was calculated for each female.
To monitor the ovarian cyclicity of the does before their introduction to the males (day 0; 10 April), blood samples were collected once per week over 3 consecutive weeks and the plasma progesterone concentration determined. The does were deemed in anoestrous if all plasma samples showed plasma progesterone concentrations of ⩽1.0 ng/ml (Chemineau et al., Reference Chemineau, Daveau, Maurice and Delgadillo1992). To monitor the ovarian response after introduction, the plasma progesterone concentration was determined twice per week. Does with plasma progesterone concentrations of ⩾1.0 ng/ml for at least two consecutive samples were deemed to have ovulated (Chemineau et al., Reference Chemineau, Daveau, Maurice and Delgadillo1992). The date of onset of the ovarian response was defined as that of the first sample with a progesterone concentration of ⩾1.0 ng/ml. Blood samples were collected by jugular venipuncture in tubes containing 10 µl heparin, and plasma obtained by centrifugation at 3500×g for 30 min. This was stored at −20°C until analysis. Plasma progesterone was determined using a commercial ELISA kit (Ridgeway Science Ltd, Gloucester, UK) in accordance with the manufacturer’s instructions. The sensitivity of the assay was 0.28 ng/ml. Intra- and inter-assay CV for sample pools of 0.5 and 1 ng/ml were 7.1%, 6.5%, and 7.9% and 9.1%, respectively.
The ovulation rate was assessed by the number of corpora lutea observed by transrectal ultrasonography conducted 6 to 8 days after the detection of oestrus. The procedure was performed using an Aloka SSD-500 (Ecotron, Madrid, Spain) apparatus connected to a 7.5 MHz linear probe.
Fecundity, fertility and productivity
Fecundity (percentage of pregnant does/does mounted by the males) was determined via transrectal ultrasonography on day 45 after mounting (Schrick et al., Reference Schrick, Surface, Pritchard, Dailey, Townsend and Inskeep1993). Fertility (percentage of does kidding per doe serviced), prolificacy (number of kids born per doe kidding) and productivity (number of kids born per doe serviced) were also determined (Caravaca et al., Reference Caravaca, Castel Genís, Guzmán, Delgado, Mena, Alcalde and González1999).
Statistical analyses
Data are presented as means±standard error. The weekly values for BW, BC, the testosterone concentration and the twice-weekly values for the progesterone concentration, were examined by ANOVA with time as a repeated measure, with doe age and buck photoperiod treatment as the main factors. The Tukey test was used to detect differences between groups and between weeks. The difference in testosterone concentration each week between the CONTROL and PHOTO bucks was analysed by one-way ANOVA.
The variables expressed as percentages (global and daily percentages of females showing ovulatory activity via the progesterone concentration, oestrous, ovulation, fecundity, fertility and females kidding) were analysed using the multinominal logistic regression.
The ovulation rate and prolificacy were compared using the Mann–Whitney U test. Productivity, and the interval between introduction and the date of females showing oestrus with ovulation were compared by ANOVA, contemplating doe age and buck photoperiod treatment as fixed effects. Significance was set at P<0.05. All analyses were performed using the STATA14 package (STATA14; StataCorp, 2015).
Results
BW and body condition scores
Body weight (BW) and BC both increased over the experimental period in both main doe groups (P<0.01; Figure 1); however, the BW and BC were higher in the 10-month-old does (P<0.01). Buck photoperiod treatment had no effect on BW or BC, nor did the interaction doe age × buck photoperiod treatment (P>0.05). Neither did the interaction doe age × buck photoperiod treatment × time have any effect on BW or BC (P>0.05).
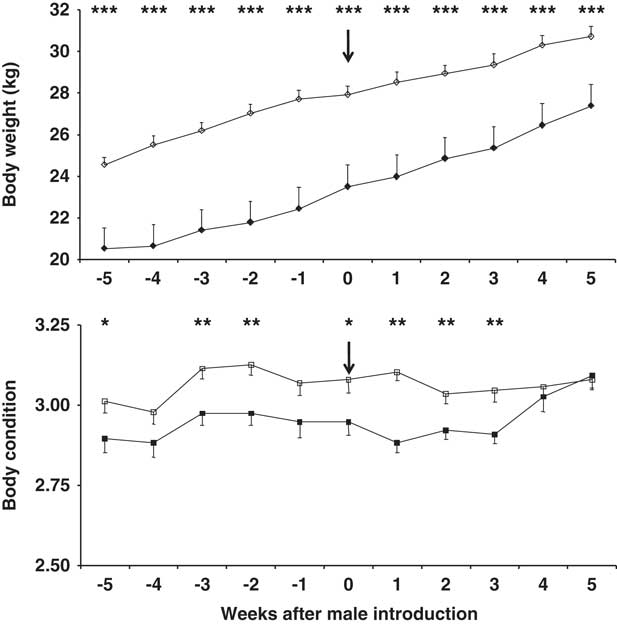
Figure 1 Change in body weight (top) and body condition score (bottom) in 7-month-old (filled symbols) and 10-month-old (blank symbols) female goats. The arrow indicates the moment when the sexes were introduced to one another. *P<0.05,**P<0.01, ***P<0.001 in the same week.
At the first ovulation, and at the first oestrus with ovulation, BW was higher in the older does, independent of the type of male to which they were exposed (P<0.05). However, no differences were seen in terms of BC between the age groups independent of the type of male present (P>0.05) (Table 1). No differences were seen in terms of BW in does exposed to CONTROL or PHOTO bucks, independent of doe age (P>0.05). In contrast BC was higher in the does exposed to CONTROL bucks, independent of doe age (P<0.05) (Table 1).
Table 1 Percentage of females showing ovulation or oestrus, BW and body condition, at those moments, intervals from male introduction to ovulation or oestrus, ovulation rate of the first oestrous, fecundity, fertility, prolificacy and productivity, in 7- and 10-month-old does exposed to photostimulated bucks (PHOTO) and to unstimulated bucks (CONTROL)
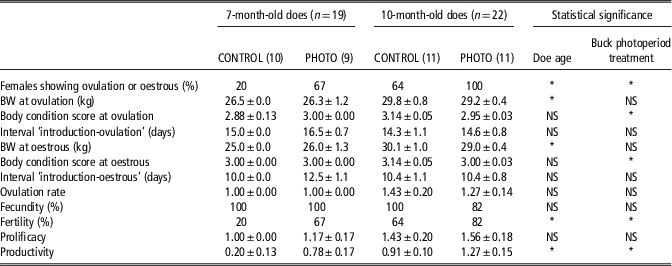
Fecundity=percentage of pregnant female goats/female goats mounted by the males, determined via transrectal ultrasonography on day 45 after mounting.
Fertility=percentage of female goats kidding per female goat serviced.
Prolificacy=number of kids born per female goat kidding.
Productivity=number of kids born per female goat serviced.
*P<0.05.
The interaction doe age × buck photoperiod treatment had no significant (NS) effect on any variable (P>0.05).
Reproductive response to the male effect
The interaction doe age × buck photoperiod treatment had no effect on any of the reproductive variables studied. The percentage of females showing ovulation via the elevation of the progesterone concentration, and oestrus with ovulation, was higher in the 10-month-old than in the 7-month-old does as a whole, independent of the type of male to which they were exposed (82% v. 42%, respectively; P<0.01). Irrespective of their age, the percentage of females showing ovulation or oestrus of the does exposed to PHOTO bucks was greater than that shown by those exposed to CONTROL bucks (85% v. 43% for the PHOTO and CONTROL male-exposed does, respectively; P<0.01) (Table 1).
The fertility of the 10-month-old does was greater than that of the 7-month-old does as a whole, independent of the type of male to which they were exposed (73% v. 42%, respectively; P<0.05). Irrespective of their age, the fertility of the females exposed to the PHOTO bucks was greater than that shown by those exposed to the CONTROL bucks (75% v. 43% for the PHOTO and CONTROL male-exposed does, respectively; P<0.05) (Table 1).
The productivity of the 10-month-old does was greater than that of the 7-month-old does as a whole, independent of the type of male to which they were exposed (1.09±0.17 kids born per doe serviced v. 0.47±0.14 goat kids born per doe serviced; P<0.05). Irrespective of their age, the productivity of the females exposed to the PHOTO bucks was greater than that shown by those exposed to the CONTROL bucks (1.05±0.17 goat kids born per doe serviced v. 0.57±0.16 goat kids born per doe serviced, respectively; P<0.05) (Table 1).
The other variables such as the intervals ‘introduction-first ovulation’ and ‘introduction-first oestrus’, the ovulation rate at first oestrus, fecundity and prolificacy, were not influenced by either doe age or buck photoperiod treatment, or their interaction.
The interval between introduction and the first increase in the progesterone concentration, or the first detected oestrous, was not influenced by doe age (P>0.05), nor by buck photoperiod treatment (P>0.05), nor by the interaction between them (P>0.05). The interval ‘introduction-first observed oestrous’ was shorter than the interval ‘introduction-first elevation of the progesterone concentration’ (10.9±0.5 days v. 15.0±0.5 days, respectively; P<0.001).
From day 14 after introduction, the percentage of does showing elevated progesterone was higher among the 10-month-old than the 7-month-old animals, independent of the type of male to which they were exposed (P<0.05) (Figure 2). From day 18 after introduction, the percentage of does showing elevated progesterone was higher among those exposed to the PHOTO males than among those exposed to the CONTROL males (P<0.05) (Figure 2). From day 11 after introduction, the percentage of does showing oestrus was higher among the 10-month olds than in the 7-month olds as a whole, independent of the type of male to which they were exposed (P<0.05) (Figure 2). Finally, from day 14 after introduction, the percentage of does showing oestrus was higher among those exposed to the PHOTO bucks than among those exposed to the CONTROL bucks (P<0.05) (Figure 2). The interaction doe age × buck photoperiod treatment had no effect on any of the above variables (P>0.05).
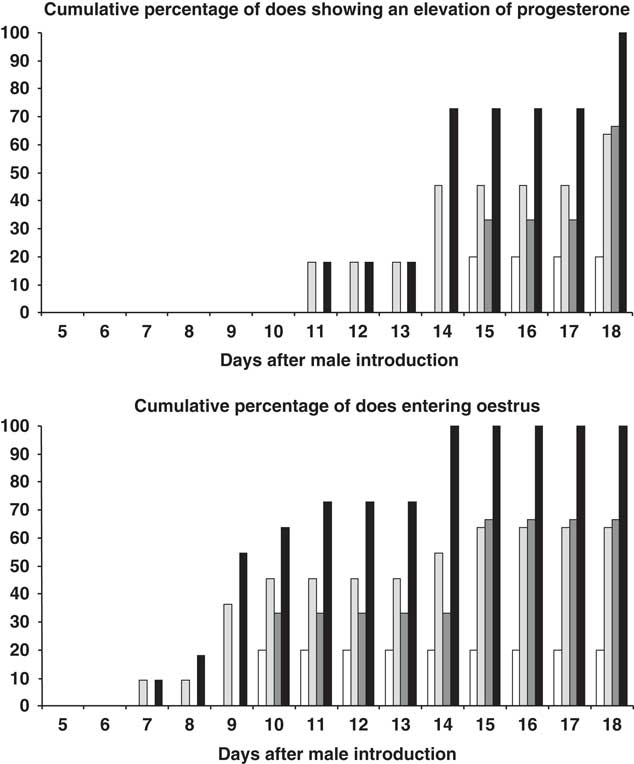
Figure 2 Top: Cumulative percentage (%) female goats showing a progesterone concentration of ⩾1 ng/ml or bottom: female goats showing oestrous of 7-month old after exposure to bucks exposed to natural changes in day length over the whole experiment (CONTROL bucks (◻), and to bucks submitted to a period of 3 months of long days, and then to the natural photoperiod (PHOTO bucks, ![]() ); and 10-month-old female goats after exposure to CONTROL bucks (
); and 10-month-old female goats after exposure to CONTROL bucks (![]() ), and to PHOTO bucks (■).
), and to PHOTO bucks (■).
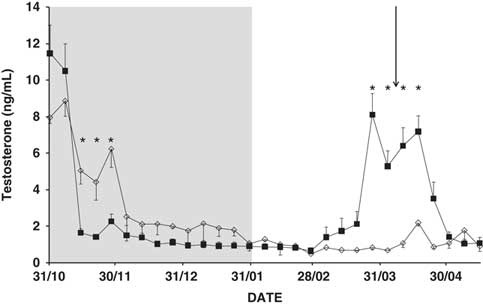
Testosterone concentrations
Time, and the interaction time × buck photoperiod treatment, were found to influence testosterone concentrations (P<0.01; Figure 3). The concentration decreased rapidly after the start of the photoperiod treatment (P<0.05), but began to increase 7 weeks after its end, and did so for about 4 weeks. In contrast, the CONTROL bucks showed very low testosterone concentrations at the end of November and thereafter (P<0.05; Figure 3). Neither doe age, nor the interaction doe age × buck photoperiod treatment, nor the interaction time × doe age × buck photoperiod treatment, had any effect on the testosterone concentration (P>0.05).

Figure 3 Mean change in plasma testosterone concentration (ng/ml) in bucks submitted to a period of 3 months of long days (grey area), and then to the natural photoperiod (PHOTO bucks, ■) or to natural changes in day length over the whole experiment (CONTROL bucks, ◊). *P<0.05 in the same week. The arrow indicates the moment when the sexes were introduced to one another.
Discussion
The present results show that doe age and the sexual activity of males are important factors to bear in mind when seeking the maximum response to the male effect in spring. Better reproductive responses and performances were observed in the 10-month-old does, and in those exposed to PHOTO bucks. These results support the hypothesis that photostimulated males can induce an intense reproductive response, and increase reproductive performance, even in very young does.
Autumn-born does of the Spanish Blanca Andaluza (Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015), Payoya (Zarazaga et al., Reference Zarazaga, Guzmán, Domínguez, Pérez, Prieto and Sánchez2009) and Murciano-Granadina (Pérez-Fuentes et al., Reference Pérez-Fuentes, Lleó-Casanova and Pérez-Picazo1999) breeds usually enter puberty in the following breeding season (i.e. the next autumn). The mean onset of puberty in Blanca Andaluza does is at 317.2±5.1 days after birth, with BW and BC having a clear influence on the exact time (Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015). Autumn- and winter-born Payoya does show their first oestrous at 257 and 348 days of age, respectively (Zarazaga et al., Reference Zarazaga, Guzmán, Domínguez, Pérez, Prieto and Sánchez2009), whereas Murciano-Granadina does ovulate at 154.4±4.2 days of age (Pérez-Fuentes et al., Reference Pérez-Fuentes, Lleó-Casanova and Pérez-Picazo1999). In the present work, the male effect was used to induce puberty at 292 and 221 days of age for the 10- and 7-month-old does, respectively. The results indicate that inducing the male effect with photostimulated males brings about earlier puberty in does. This was true even for the present 7-month-old does, which entered their first oestrous with a mean BW of 25.8±1.0 kg and a mean BC score of 3.00±0.00. According to previous results for Blanca Andaluza does born in November, these values are appropriate for the onset of breeding activity (Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015). In the latter experiment, oestrous activity first began earlier in does with a BC of around 3.00points and a BW of >24.0 kg. November- and February-born Payoya does showed their first oestrous after reaching a BW of 28.7±0.6 kg plus a BC score of 2.73±0.04 and 23.3±0.5 kg plus a BC score of 2.64±0.05, respectively. These figures indicate that all the does in the present work had reached an adequate developmental condition for onset of puberty and, perhaps, to respond to the male effect.
The percentage of 10-month-old does that ovulated and entered oestrous was higher than for the 7-month-old does. These results were expected since the former does were older and had a greater BW; therefore, closer to the threshold for the onset of puberty. Gordon (Reference Gordon1975) indicated that puberty in farm animals requires a critical BW be reached. For goats, Smith (Reference Smith1980) reported this to be 60% to 75% of the mature BW. For Blanca Andaluza does, the critical BW for initiating puberty is ~60% of the mature BW (Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015). In the present experiment, the 7-month-old does showed their first oestrus at 25.8±1.0 kg, while the 10-month-old does did so at 29.5±0.5 kg, that is, at around 55% and 63% of their mature BW, respectively. The slightly higher BW of the 10-month-old does might explain their greater reproductive response; the hypothalamic–pituitary–ovarian axis in these animals might be better developed, leading to a higher frequency of LH pulses during the prepubertal period resulting in earlier onset of puberty (Foster et al., Reference Foster, Lemons, Jaffe and Niswender1975). Moreover, during the prepubertal period, the preovulatory gonadotrophin surge system is able to function, but it remains inactive in the absence of sustained high physiological levels of oestradiol (Foster et al., Reference Foster, Yellon and Olster1985). As a consequence, the reduced sensitivity of the axis to oestradiol originates a high-frequency rhythm of gonadotropin-releasing hormone and a sustained rise in basal LH secretion as the frequency of LH pulses increases. The first follicular phase then begins, leading to the first LH surge and ovulation (Foster and Jackson, Reference Foster and Jackson2006).
The does in contact with the PHOTO bucks showed a better percentage of females showing ovulation or oestrus irrespective of doe age. This is likely due to the intense sexual behaviour and strong odour of these bucks. The stimulatory effect of long days on the capacity of bucks to induce a male effect has been extensively documented at temperate latitudes (Chasles et al., Reference Chasles, Chesneau, Moussu, Delgadillo, Chemineau and Keller2016), under Mediterranean conditions (Zarazaga et al., Reference Zarazaga, Gatica, Celi, Guzmán and Malpaux2010) and under subtropical conditions (Flores et al., Reference Flores, Véliz, Pérez-Villanueva, Martínez de la Escalera, Chemineau, Poindron, Malpaux and Delgadillo2000). However, this is the first indication that it can stimulate a reproductive response in such young does. It might be argued that, rather than this being an effect of exposure to these PHOTO bucks, doe BW or BC might be responsible. However, the design of the present experiment means this possibility can be ruled out. First, there were no differences in BW at the first progesterone elevation point, or first oestrus, between the females exposed to the PHOTO or CONTROL bucks – and yet the reproductive response of the PHOTO buck-exposed females was stronger. In addition, the BC of the does exposed to the CONTROL bucks was actually slightly higher than that of their PHOTO buck counterparts, which in any event also surpassed the ~2.50 points required (Gallego-Calvo et al., Reference Gallego-Calvo, Gatica, Celi, Guzmán and Zarazaga2015).
The positive reproductive response of the does exposed to PHOTO bucks was confirmed by their higher fertility and productivity results. When exposed to these males, the 10-month-old does produced one kid per doe more than did the 7-month olds exposed to CONTROL bucks. These results confirm previous findings (Zarazaga et al., Reference Zarazaga, Gatica, Hernández, Gallego-Calvo, Delgadillo and Guzmán2017 and Reference Zarazaga, Gatica, Gallego-Calvo and Guzmán2018). The reason for the lack of any difference between any groups (i.e. with respect to doe age or buck photoperiod treatment) in terms of fecundity can be explained in that this variable is calculated from the number of females that become pregnant compared to those that showed signs of oestrus. In general, all the does that entered their first oestrous became pregnant, indicating that all were in optimum reproductive condition (the males too). The main question is thus the percentage of induction into puberty achievable by the male effect, rather than fecundity once that induction has been achieved.
The intervals ‘introduction-oestrous’ or ‘introduction-first elevation of the progesterone concentration’, were not modified by doe age, buck photoperiod treatment or their interaction. However, the interval ‘introduction-first oestrous behaviour’ was shorter than the interval ‘introduction-first elevation of progesterone concentration’. This result may largely be explained by the procedure used to estimate the interval to the first elevation of the progesterone concentration, that is, when the progesterone concentration reached ⩾1 ng/ml. This occurs when the corpora lutea are fully functional, some days after the start of oestrous behaviour (Zarazaga et al., Reference Zarazaga, Malpaux and Chemineau1996). The present does therefore showed a typical response to the male effect – a short ovarian cycle unaccompanied by oestrus, followed by a first oestrus accompanied by ovulation about 10 days after teasing (Chemineau, Reference Chemineau1983). However, in the present work, no short elevation of progesterone concentrations was seen to occur, probably because the twice weekly frequency of determination was insufficient to detect it.
Finally, the distribution of the daily percentage of females showing oestrous differed to that reported in other studies (Flores et al., Reference Flores, Véliz, Pérez-Villanueva, Martínez de la Escalera, Chemineau, Poindron, Malpaux and Delgadillo2000; Véliz et al., Reference Véliz, Moreno, Duarte, Vielma, Chemineau, Poindron, Malpaux and Delgadillo2002); the latter authors observed oestrus in the 1st week after introduction. However, the latter authors’ experiments were performed at subtropical latitudes and the does were all adults. Certainly, Véliz et al. (Reference Véliz, Meza-Herrera, De Santiago-Miramontes, Arellano-Rodriguez, Leyva, Rivas-Muñoz and Mellado2009) observed the distribution of females showing oestrus following the male effect to differ between multiparous and nuliparous females.
Conclusions
The present results show that, at Mediterranean latitudes, the reproductive response induced by photostimulated bucks is stronger in both 10- and 7-month-old does, than that induced by untreated bucks. Although the present PHOTO bucks advanced the onset of puberty in does of both ages, increasing their productivity, the 10-month-old does exposed to PHOTO bucks produced one kid more per doe serviced than did the 7-month-old does exposed to CONTROL bucks. Mating summer/autumn-born does with photostimulated bucks would appear to be a practical means of increasing their reproductive/productive performance.
Acknowledgements
This study was funded by Grant AGL2016-75848-R from MINECO-AEI-FEDER (Spain). Thanks are also owed to CEI CamBio for their support.
Declaration of conflicts of interest
None of the authors of this paper has any financial or personal relationship with any other person or organisation that might inappropriately influence or bias the content of the paper.
Ethics statement
All procedures were performed by trained personnel in strict accordance with Spanish guidelines for the protection of experimental animals (RD 53/2013), and in agreement with European Union Directive 86/609.
Software and data repository resources
None of the data were deposited in an official repository.