Introduction
Primary squamous cell carcinoma (SCC) of the external auditory canal is rare with a reported worldwide annual incidence of one in a million.Reference Takenaka, Cho, Nakahara, Yamamoto, Yasui and Inohara1,Reference Crabtree, Britton and Pierce2 Squamous cell carcinoma is the most common histological subtype, accounting for less than 0.2 per cent of all the head and neck tumours.Reference Zanoletti, Lovato, Stritoni, Martini, Mazzoni and Marioni3 Due to the complex anatomical location of these tumours and their invasive nature, prognosis remains poor, especially in advanced cases. These tumours are also usually misdiagnosed in the early stages owing to the similarity of symptoms to those of benign diseases.Reference Zhang, Dai and Wang4 Common risk factors include chronic middle-ear diseases and chronic otitis externa. The causal association between these two was first described by WhiteheadReference Whitehead5 in 1908.
There is a lack of consensus in the literature in terms of the diagnosis, staging and therapeutic approach to primary SCC of the external auditory canal when compared to other head and neck SCC. This is mainly due to the rarity of these tumours. Currently available therapeutic options include surgery, radiotherapy, chemotherapy and possible immunotherapy in the future. Presence of nodal metastasis, advanced T-stage at presentation, histological subtype of SCC, facial paralysis, dural involvement and positive margins were found to be negative prognostic factors resulting in a poor survival rate in the majority of the reported series.Reference Madsen, Gundgaard, Hoff, Marre, Holmboe and Knap6,Reference Moody, Hirsch and Myers7 The classification and segregation of T-stage has often been debated, and Higgins et al. discuss the different classification systems, notably the Pittsburgh staging system classification (Pittsburgh-1990 and Pittsburgh-2000) with no consensus in the management of this rare disease.Reference Moody, Hirsch and Myers7,Reference Higgins and Antonio8
In this study, we did a systematic review of the available existing literature regarding the survival outcome based on the T-staging of the Pittsburgh classification system. The role of surgery, radiotherapy (RT) or chemotherapy is not clearly defined, and international practice remains varied. This analysis provides the ground for a new classification and staging system for primary SCC of the external auditory canal with higher prognostic accuracy and greater simplicity. The implementation of this simple classification should facilitate prospective clinical trials and evaluation to help standardise the management of primary SCC of the external auditory canal.
Materials and methods
Information source and search strategy
We searched Medline using the PubMed database and Ovid for articles on external auditory canal SCC published up to January 2018. For the PubMed search, we used the following medical subject headings (‘MeSH’) and free-text terms: ‘squamous cell carcinoma’ AND ‘external auditory canal’ AND ‘surgery’ AND ‘prognosis’. Filters were applied to restrict it to human studies and English language.
Study selection
All retrospective and prospective cohorts with more than 15 subjects were studied. Studies were eligible if they met the following inclusion criteria: (1) reported tumour characteristics such as histological confirmation of SCC and site of primary origin at the external auditory canal, (2) use of the Pittsburgh classification to stage tumours, (3) the outcome of the studies at least provided information on overall survival at two years and (4) studies with survival outcome by staging. The modified Pittsburgh-2000 classification, reported by Moody et al.Reference Moody, Hirsch and Myers7 and Higgins et al.Reference Higgins and Antonio8 is shown in Table 1 in the supplementary material, available on The Journal of Laryngology & Otology website. We excluded case reports and abstracts without full articles or patient series with less than 15 patients.
Data abstraction
The title and abstract of each study were reviewed by an investigator under the supervision of a second investigator. The two authors independently assessed eligibility. Differences were resolved by consensus. Pertinent studies were screened with rigor to identify the various treatments used, follow up, methodological quality, patient demographic data and the outcomes by staging. Quality of evidence was assessed using the Grading of recommendation, Assessment, Development and Evaluation (‘GRADE’) working group system. The papers were analysed to ensure there were no duplicate data included.
Statistical analysis
Proportions of survival at five years were calculated from the relevant numerator and denominator when data permitted. The overall proportions of survival at five years were derived using meta-analysis techniques and presented along with 95 per cent confidence intervals (CIs) using a Freeman–Tukey transformation to calculate the weighted summary proportion under the fixed and random effects model.Reference Higgins and Antonio8 Forest plots were created, showing the individual study results, weights of the individual study and overall weighted proportions together with 95 per cent CIs. Forest plots were also created to explore differences in survival rates at five years according to tumour stages. Analyses were performed with MedCalc statistical software using Freeman–Tukey transformation under the fixed and random effects model.Reference DerSimonian and Laird9 Proportions were analysed using Cochran's Q test to detect heterogeneity. A p-value less than 0.05 was considered as significant, thus showing heterogeneity. The I-squared (I2) statistic was also calculated to measure the percentage of variability between summary proportions that were due to heterogeneity rather than chance. An I2 more than 50 per cent was considered to have substantial heterogeneity. When heterogeneity was detected, the results of random effects model were considered whereas when no heterogeneity was detected the results of fixed effect model was considered. Publication bias was examined by constructing a funnel plot.
Cluster analysis
Partition around medoids clustering is an heuristic method to partitioning ‘n’ data points into ‘k’ clusters. Using MATLAB, a k-means clustering script was written in R programming language to analyse our multivariable individual patient data from the cohort. Variables analysed included tumour–node–metastasis (TNM) status and disease-free survival with or without nodes. From the studies, individual patients (n = 61) with full data were gathered and a comma-separated values (‘csv’) file was created. The script, using the comma-separated values file as raw data, clustered the patients into k = (1 to 10) clusters over 1000 iterations. The number of clusters was computed using the elbow method, which calculated the within-cluster sum of squares and plots over number of clusters. The ‘elbow’ in the two-dimensional plot and the silhouette width identified the ideal number of clusters. Once the ideal number of clusters was determined, n = 61 data points were clustered into k = 4 clusters (Table 2 in the supplementary material, available on The Journal of Laryngology & Otology website).
Results
Literature search
The search strategy identified 234 publications (Figure 1). Thirty-three publications were retrieved for full-text analysis based on the title and abstract. After applying the inclusion criteria, 10 articles were selected with an overall survival rate of a minimum of 2 years. Finally, eight series studies with an overall survival rate of five years were included in this review. A systematic review was done for the survival outcome based on T-stage. Each selected series study had at least 15 patients.

Fig. 1. Flowchart diagram of the study selection process. Studies excluded (n = 25) were due to insufficient data, overlapping data, mixed pathologies, being case reports only or a case series less than 20 patients.
Study characteristics
Tables 1 and 2 show population characteristics and the treatment strategies of all the retrieved studies. All 8 studies were retrospective and were published between 1994 and 2018.
Table 1. Epidemiology and treatment strategies

*n = 437; IQR = interquartile range
Table 2. Summary of studies showing characteristics of patients and disease

SCC = squamous cell carcinoma; M = Male; F = female; OSR = overall survival rate
Survival analyses
Eight studies indicated survival rates at five years. The pooled proportion of survivors at 5 years was 53.0 per cent (95 per cent CI: 44.6–61.4; Table 2).Reference Higgins and Antonio8,Reference Austin, Stewart and Fawzi10–Reference Ogawa, Nakamura, Hatano, Suzuki, Ito and Murayama17 Significant statistical heterogeneity (0.065/I2, 39 per cent) was found for the 5-year survival proportion (p Het = 0.006, I2 = 65 per cent). This heterogeneity could be explained by the different stages of the tumours in the included articles and the difference in management strategy. The funnel plot was symmetric suggesting symmetry in the data and low likelihood of publication bias (see Figure 1 in the supplementary material, available on The Journal of Laryngology & Otology website).
T-stages analysis
The pooled proportion of survivors at 5 years for T1, T2 and T1 and T2 tumours combined was 88.4 per cent (95 per cent CI: 66.0–99.5)Reference Higgins and Antonio8,Reference Mazzoni, Danesi and Zanoletti11–Reference Yin, Ishikawa, Honda, Arakawa, Harabuchi and Nagabashi14 (Figure 2a), 88.6 per cent (95 per cent CI: 68.8–99.1)Reference Austin, Stewart and Fawzi10–Reference Hashi, Shirato, Omatsu, Kagei, Nishioka and Hashimoto15 (Figure 2b) and 84.5 per cent (95 per cent CI: 70.2–94.7)Reference DerSimonian and Laird9–Reference Ogawa, Nakamura, Hatano, Suzuki, Ito and Murayama17 (Figure 2c). The pooled proportion of survivors at 5 years for T3, T4 and T3 and T4 tumours combined was 53.3 per cent (95 per cent CI: 41.6–64.8)Reference Austin, Stewart and Fawzi10,Reference Lobo, Llorente and Suarez12–Reference Pemberton, Swindell and Sykes16 (Figure 2d), 26.8 per cent (95 per cent CI: 18.5–36.6)Reference Mazzoni, Danesi and Zanoletti11–Reference Prabhu, Hinerman, Indelicato and Morris13,Reference Hashi, Shirato, Omatsu, Kagei, Nishioka and Hashimoto15 (Figure 2e) and 40.4 per cent (95 per cent CI: 34.6–6.4)Reference DerSimonian and Laird9,Reference Austin, Stewart and Fawzi10,Reference Lobo, Llorente and Suarez12,Reference Prabhu, Hinerman, Indelicato and Morris13,Reference Hashi, Shirato, Omatsu, Kagei, Nishioka and Hashimoto15,Reference Ogawa, Nakamura, Hatano, Suzuki, Ito and Murayama17 (Figure 2f).

Fig. 2. Forest plot for pooled proportion of survivors at five years according to the T-stages: (a) T1 tumours, (b) T2 tumours, (c) T1 + T2 tumours, (d) T3 tumours, (e) T4 tumours, (f) T3 + T4 tumours and (g) all stages.
Cluster analysis results
A total of 61 patients were grouped into four clusters in a multivariable analysis, and the results were correlated with the disease-free survival of the patients. Variables analysed included TNM status and disease-free survival. There were 17 patients in cluster 1 included (T1 without node, n = 0), 16 patients in cluster 2 (T2 with nodes, n = 3), 13 patients in cluster 3 (T3 with no nodes, n = 0) and finally 15 patients in cluster 4 (T4 with nodes, n = 3). Table 3 in the supplementary material (available on The Journal of Laryngology & Otology website) shows the survival based on the clusters, and there was a monotone decrease as the cluster number moved from 1 to 4. Although there was an increase in silhouette width with increase in clusters, clustering into 4 gives a better clarity. Nodal positivity negatively influenced the survival rate based on this cluster analysis irrespective of the primary T-stage. The mean survival of cluster 3 was better than cluster 2, which was characterised by its nodal positivity (54.36 versus 52.58).
Discussion
The pre-existing clinical studies showed great variations in pre-operative staging and post-operative outcome parameters. The only consistent finding we could gather from all patients was T-stage based overall survival rate at five years. Overall, we could not make any definitive statements about the choice and impact of various treatments on the prognosis of the disease as none of these case series gave the treatment modality based on the T-staging. Fifty (11.4 per cent) patients were treated with surgical resection alone, and 190 (43.5 per cent) were treated with RT alone highlighting the heterogeneity in the treatment modalities for primary SCC of the external auditory canal (Table 1). Pemberton et al. reported a 5-year overall survival rate of 40 per cent in their series of 123 patients treated with primary RT alone,Reference Pemberton, Swindell and Sykes16 which included T1–T4 lesions. This clearly shows that no treatment algorithm was utilised for this rare disease. However, excellent overall survival associated with low-grade T-stage emphasises the importance of early diagnosis when the lesion is still confined to the soft tissues of the external auditory canal. Long standing chronic external otitis with frequent history of otorrhoea, otalgia and bleeding should not be overlooked as warning signs for an early diagnosis, and repeated biopsies might be necessary to exclude malignancy in these patients. However, advanced T-stage disease will warrant a multidisciplinary team approach comprising of head and neck surgeons, radiation oncologists, medical oncologists and neurosurgeons.
T1 and T2 tumours and survival
The present systematic review of primary SCC of the external auditory canal showed an excellent survival outcome at five years for T1 and T2 lesions. The pooled proportion of survivors at 5 years for T1, T2 and combined T1 and T2 tumours was 88.4 per cent, 88.6 per cent and 84.5 per cent. Oya et al. as well as other studiesReference Oya, Takenaka, Takemura, Ashida, Shimizu and Kitamura18–Reference Ihler, Koopmann, Weiss, Drog, Durisin and Christiansen22 in their systematic review of the literature reported a 5-year overall survival rate of 77 per cent for early stage (stage I and II according to Pittsburgh classification) primary SCC of the external auditory canal.
In our present systematic review, the overall survival rates are marginally superior to those shown in the analysis by Oya et al.Reference Oya, Takenaka, Takemura, Ashida, Shimizu and Kitamura18 Generally, the prognosis of patients with T1 or T2 lesions, treated by surgery or RT or both, is very good as reported in the literature.Reference Bacciu, Clemente, Piccirillo, Ferrari and Sanna23–Reference Moore, Deschler, McKenna, Varvares and Lin26 This also stresses the importance of treating the patient when the lesion is still confined to the soft tissues of the external auditory canal. Hashi et al.Reference Hashi, Shirato, Omatsu, Kagei, Nishioka and Hashimoto15 and Ogawa et al.Reference Ogawa, Nakamura, Hatano, Suzuki, Ito and Murayama17 reported 5-year overall survival rates at 100 per cent and 83 per cent, respectively, for T1 tumours treated with RT alone.
Although RT represents a valuable alternative with good survival rates, it may not represent the eligible primary resection treatment for these T1 and T2 lesions. The main reason for this is that any recurrence at this site will make the secondary surgical treatment difficult. The high-quality outcomes achieved with surgery with smaller lesions are related to the possibility of ensuring a complete en-bloc resection with macroscopically clear margins. Bacciu et al. treated T1–T2 lesions with surgery and T3–T4 lesions with additional post-operative RT resulting in an overall survival rate of 69 per cent in the whole series.Reference Moody, Hirsch and Myers7,Reference Bacciu, Clemente, Piccirillo, Ferrari and Sanna23,Reference Nakagawa, Kumamoto, Natori, Shiratsuchi, Toh and Kakazu27 Recurrence seems to be a major setback in patients with primary SCC of the external auditory canal even after curative resection of the tumour ranging from 5–19 per cent in T1 and T2 tumours.Reference Pemberton, Swindell and Sykes16,Reference Bacciu, Clemente, Piccirillo, Ferrari and Sanna23
T3 and T4 tumours and survival
The systematic review of the literature by Higgins et al Reference Higgins and Antonio8 reported on a 5-year overall survival rate of 57.5 per cent and 22.9 per cent for T3 and T4 primary SCC of the external auditory canal, respectively. The classification and segregation of T3 and T4 cases have often been debated, especially based on facial nerve paresis. Higgins et al. discuss the different classification systems, notably the Pittsburgh staging system, (Pittsburgh-1990 and Pittsburgh-2000) and show that patients with primary SCC of the external auditory canal presenting with facial palsy have Kaplan–Meier curves that parallel closely Pittsburgh-2000 T4 patients.Reference Higgins and Antonio8 This may explain why our systematic review on some T3 and T4 cohorts will more closely follow the median 5-year overall survival rate of a more advanced or less advanced stage.
As discussed in the case of T1 and T2 stage primary SCC of the external auditory canal, the lack of a standardised treatment protocol is also a limitation for more advanced T3 and T4 stage cases. The general consensus for advanced stage primary SCC of the external auditory canal consists of subtotal temporal bone resection or total temporal bone resection combined with post-operative RT.Reference Conley and Novack28–Reference Lassig, Spector, Soliman and El-Kashlan31 Takenaka et al. discussed the different treatment protocols offered by different case series and compared their 180-month survival.Reference Takenaka, Cho, Nakahara, Yamamoto, Yasui and Inohara1,Reference Ueda, Kurita, Matsuda, Ito and Nakashima32–Reference Cristalli, Manciocco, Pichi, Marucci, Arcangeli and Telera34 The crux of the problem with advanced stage disease is evaluating and balancing the morbidity of the disease because of the extent of local invasion versus the morbidity of the post-operative or post-surgical complications. Interestingly, overall survival rate of less invasive surgical procedures such as lateral temporal bone resection remains higher until 100 months post-operatively than subtotal temporal bone resection or total temporal bone resection, but then becomes inferior afterwards. These statistics are to be considered given the clinical status and co-morbidities of a patient.Reference Takenaka, Cho, Nakahara, Yamamoto, Yasui and Inohara1 Takenaka et al. also showed in their systematic review that the hazard ratio of pre-operative chemo-RT and surgery is 0.18 with a 95 per cent CI of 0.01–0.88 and a p-value of 0.030.Reference Takenaka, Cho, Nakahara, Yamamoto, Yasui and Inohara1,Reference Nakagawa, Kumamoto, Natori, Shiratsuchi, Toh and Kakazu27,Reference Shiga, Ogawa, Maki, Amano and Kobayashi35 In the future, the conduction of randomised controlled trials may be warranted.
Nodal disease and overall survival
The incidence of cervical node metastasis ranges from 10–23 per cent.Reference Moffat, Wagstaff and Hardy36 From the eight studies, we were able to construct and compare disease-free survival in nodal positive and nodal negative disease in only three series. In our systematic review, 12.35 per cent (Table 2) of patients had positive nodal disease although nodal staging was often not reported in many series. Using a cluster algorithm, these patients did correlate with disease-free survival. When we cluster the data based on the TNM staging and compare the disease-free survival of patients with or without nodes, they had an average disease-free survival of 13.92 months versus 32 months. Nodal positivity negatively influenced the survival rate.
Gidley et al Reference Gidley, Thompson, Roberts, DeMonte and Hanna37 reported that level I and II are the most commonly involved lymph node echelons. Literature strongly supports neck dissection in cases where there is clinical or radiological evidence of neck nodes or parotid involvement.Reference Zanoletti, Marioni, Stritoni, Lionello, Giacomelli and Martini38,Reference Nyrop and Grontved39 However, there are reported rates of 4.5 per cent to 31.8 per cent of positive nodes even after neck dissection, with a cumulative rate of 17.7 per cent.Reference Moffat, Wagstaff and Hardy36 Mazzoni et al Reference Mazzoni, Danesi and Zanoletti11 found 27 per cent of node positive cases, including four patients with clinically positive nodes and five with clinically negative nodes. The rate of micro-metastases in clinically negative necks was 17 per cent. Mazzoni et al Reference Mazzoni, Danesi and Zanoletti11 and Ogawa et al Reference Ogawa, Nakamura, Hatano, Suzuki, Ito and Murayama17 found a disease-free survival of 62.5 per cent and 57 per cent in nodal negative cases and 22 per cent and 25 per cent in nodal positive cases. According to Lobo et al.Reference Lobo, Llorente and Suarez12,Reference Barrs40,Reference Devaney, Boschman, Willard, Ferlito and Rinaldo41 the 5-year overall survival rate in N0 patients was 61 per cent, whereas in N1 patients it was 0 per cent (statistically significant difference, p < 0.006). Hence, nodal disease emerged as a variable for poor prognosis with a higher rate of local recurrences, warranting a more aggressive treatment. Based on our cluster analysis, nodal positivity negatively influenced the survival rate.
Histological factors to consider
We selected only primary SCC of the external auditory canal, and other tumours were excluded from the analysis on overall survival rate. Biochemical factors and the expression of different tumoral proteins, such as laminin-5 gamma2, epidermal growth factor and p53 tumour suppressor protein, will further subdivide and influence the overall survival rate of even the advanced stage cancers.Reference Morita, Nakamaru, Homma, Yasukawa, Hatakeyama and Sakashita42 Okado et al.Reference Okado, Aoki, Hamasaki, Koga, Sueta and Shiratsuchi43 showed that disease-free survival in T3 and T4 cases is significantly influenced by the expression of laminin-5 gamma2 (0.6 vs 0.02, p = 0.048).
Facial paralysis, dura mater and parotid gland involvement
The landmark papers by Higgins et al Reference Higgins and Antonio8 showed the importance of facial nerve paralysis on overall prognosis. Kaplan–Meier survival curves practically superimpose overall survival rate curves of Pittsburgh-2000 T4 tumours. Lobo et al Reference Mazzoni, Danesi and Zanoletti11 described the impact of facial nerve paralysis as a negative prognostic factor in the overall survival rate. In fact, the 5-year overall survival rate of patients with or without facial paralysis were 0 per cent and 47 per cent, respectively. Five year overall survival rate of 25 patients with or without facial nerve palsy were 19.1 per cent and 56.2 per cent, respectively, as reported by Higgins et al.Reference Higgins and Antonio8 Lobo et al. have also described the poor survival associated with intracranial involvement. Nodal involvement (cervical and parotid nodes) along with dural invasion was also described as a negative prognostic factor to overall survival rate by Lionello et al.Reference Lionello, Stritoni, Facciolo, Staffieri, Martini and Mazzoni44–Reference Hosokawa, Mizuta, Takahashi, Okamura, Takizawa and Hosokawa46
Some authors recommend superficial parotidectomy along with lateral temporal bone resection even in early stages of primary SCC of the external auditory canal.Reference Kunst, Lavieille and Marres47,Reference Morris, Mehra, Shah, Bilsky, Selesnick and Kraus48 The closeness to the external auditory canal and the weak resistance offered by the preauricular tissue makes it susceptible to tumour invasion.Reference Morris, Mehra, Shah, Bilsky, Selesnick and Kraus48 Even in the absence of pre-operative radiological evidence of parotid involvement, the parotid still could be involved in the histopathology as reported by Zhang et al.Reference Zhang, Dai and Wang4 A superficial parotidectomy should be considered in all cases of primary SCC of the external auditory canal due to the fact that parotid nodes are the first draining nodes, and hence at high risk for metastasis.Reference Morris, Mehra, Shah, Bilsky, Selesnick and Kraus48 Given its anatomical location, the involvement of the parotid gland should be investigated as a prognostic factor in overall survival in the future studies. In our systematic review of 437 patients, only 8 patients with facial palsy and 10 with intracranial extension were clearly described. Parotid involvement was not systematically described in these studies. Of course, advanced T-stage patients (T3–T4) could have had one of these findings but this was only seldom clearly reported.
Limitations of existing studies and staging
The scope of this study was limited in several ways. The main limitations were related to insufficient data on the available studies utilising strict inclusion criteria. A specific analysis of predictors of prognosis was not performed in the majority of studies and neither tumour characteristics nor treatment details were shown to correlate with overall survival rate. Hence, we could not analyse the prognosis of T-stage lesions based on treatment modality due to its extreme heterogeneity.
The methodological drawbacks associated with these retrospective studies included lack of standardisation in the treatment strategies, staging systems and small patient cohorts. Mazzoni et al. have reported that tumours spreading to periauricular soft tissues and parotid space had a different effect on outcome than when they extended to the mastoid and deeper parts of the temporal bone. Tumours staged as T4 due to periauricular or anterior growth and treated with lateral temporal bone resection had a better outcome than T4 cases extending posteriorly, medially and inferiorly treated with subtotal temporal bone resection. For the eight T4 cases extending anteriorly, the disease-free survival rate was 62.5 per cent (5 of 8), and disease-specific survival rate was 75 per cent (6 of 8), while the disease-free survival was 0 per cent (0 of 13) and disease-specific survival was 15 per cent (2 of 13) for the 13 T4 cases extending medially or posteriorly.Reference Mazzoni, Danesi and Zanoletti11 Among 12 cases with extensive soft tissue versus bone involvement, Ito et al Reference Ito, Hatano and Yoshizaki49 reported that only extensive bone involvement correlated with a worse prognosis. This difference may raise the question of whether the current staging of T4 tumours needs to be revised.Reference Mazzoni, Danesi and Zanoletti11 This current TNM staging system is largely T-status dependent with only minimal focus on nodal status.
Currently, according to the Pittsburgh-2000 classification system, T1N0 SCC is stage I, T2N0 is stage II, T3N0 and T1N+ are stage III and T4N0 with T2-4N+ are considered stage IV (Table 1 in the supplementary material). This current overall staging system is again largely determined by the T-status dictated with only minimal focus on nodal status. However, the prognosis is related to the overall staging rather than the actual T-status. With further detail on neck nodal status, correlations on overall survival could be extrapolated in our systematic review, suggesting a more nodal weighted staging system in the future.
Proposal of a new classification
In this systematic review of primary SCC of the external auditory canal, we could not delineate the ideal treatment option that was decided by the initial Pittsburgh classification. Only three studies (AustinReference Austin, Stewart and Fawzi10, LoboReference Lobo, Llorente and Suarez12 and HashiReference Hashi, Shirato, Omatsu, Kagei, Nishioka and Hashimoto15 et al.) had complete individual data (61 patients). We now propose a new classification, which divides primary SCC of the external auditory canal into group A, B, C and D, similar to the Kadish classification of olfactory neuroblastomas.Reference Kadish, Goodman and Wang50
We combined the T1 and T2 tumours as group A in which the tumour is limited to the soft tissues of the external auditory canal with or without partial bony erosion. Generally, the prognosis of these patients whether treated by surgery, RT or both is very good as reported in the literature.Reference Bacciu, Clemente, Piccirillo, Ferrari and Sanna23 Group B includes tumour eroding full thickness of osseous external auditory canal or tumour involving any of the following: middle ear, mastoid, parotid with or without parotid nodes, infratemporal fossa, temporo-mandibular joint or both (with or without facial palsy) where it's considered still operable. Group C includes tumours of the external auditory canal with intracranial extension which is advanced and inoperable. Nodal involvement was shown to be a sign of tumour aggressivenessReference Moody, Hirsch and Myers7 and hence additional cervical nodal involvement is included in group D (Table 3).
Table 3. Newly proposed classification of squamous cell carcinoma of the external auditory canal
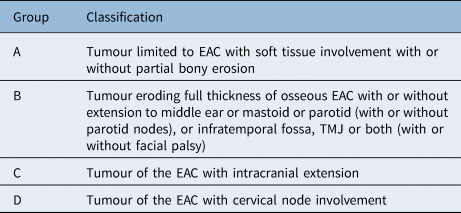
EAC = external auditory canal; TMJ = temporo-mandibular joint
The cluster analysis supported this new classification, which is shown in Table 3 in the supplementary material. The cluster analysis allowed computational allocation of data points into mathematically divided clusters. Because of the absence of individual patient parameters in many of the studies, the clustering could only be conducted on a small group of the aggregated patient pool. Since k means clustering is an heuristic method, the optimal solution or grouping of patients based on individual diagnoses parameters were limited by the number of iterations of the algorithms, with different solutions around the local optimum of the data set. However, it did provide an objective oversight of the way we grouped patients. The need for more individual data points is warranted.
Conclusion
There is lack of consensus in the literature regarding the reporting of data and therapeutic approach to primary SCC of the external auditory canal. Our systematic review of multicentre case series of primary SCC of the external auditory canal suggests that T1 and T2 tumours according to Pittsburgh's classification have a similar survival outcome. The prognosis of T3 and T4 patients remains poor, which prompts the need for more accurate prognostic calculators that can improve individualised outcomes for these patients. With further detail on neck nodal status, correlations on overall survival could be extrapolated in our cluster analysis, suggesting a more nodal weighted staging system in the future. Hence we propose a practical classification that accurately stratifies patients, and its prospective clinical application could help establish a standardised management protocol for this rare tumour.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022215121000323.
Competing interests
None declared









