Introduced predators can drive local populations to extinction (Bradford, Reference Bradford1991; Bradford et al., Reference Bradford, Graber and Tabatabai1994; Gamradt & Kats, Reference Gamradt and Kats1996; Matthews et al., Reference Matthews, Pope, Peisler and Knapp2001) and native species that inhabit permanent wetlands, such as amphibians, are frequently the most affected by introduced predators (Wellborn & Skelly, Reference Wellborn, Skelly and Werner1996; Kats & Ferrer, Reference Kats and Ferrer2003). In Patagonia, Argentina, several endemic amphibians are potentially vulnerable to, or have been affected by, introduced species (Perotti et al., Reference Perotti, Diéguez and Jara2005; Ortubay et al., Reference Ortubay, Cussac, Battini, Barriga, Aigo and Alonso2006). An emblematic example is Atelognathus patagonicus, an amphibian endemic to the Laguna Blanca wetland system, Neuquén province (Cei, Reference Cei1980). This species, categorized as Endangered on the IUCN Red List (IUCN, 2008) and the only native aquatic vertebrate in this lagoon system, was formerly abundant in the 1,667 ha Laguna Blanca, the main lagoon (Péfaur & Duellman, Reference Péfaur and Duellman1980; Administración de Parques Nacionales, 1993). However, it is now restricted to small lagoons inside and outside the National Park (Fox et al., Reference Fox, Yoshioka, Cuello and Úbeda2005; Cuello & Perotti, Reference Cuello and Perotti2006). Hypotheses to explain why A. patagonicus no longer occur in Laguna Blanca include habitat loss, competition, and predation by perch Percichthys colhuapiensis (Fox et al., Reference Fox, Yoshioka, Cuello and Úbeda2005; Cuello & Perotti, Reference Cuello and Perotti2006; Ortubay et al., Reference Ortubay, Cussac, Battini, Barriga, Aigo and Alonso2006), which were introduced to Laguna Blanca and other small lagoons that are naturally fishless (Ortubay et al., Reference Ortubay, Cussac, Battini, Barriga, Aigo and Alonso2006).
The first step in evaluating the potential impact of P. colhuapiensis on A. patagonicus is to compare presence/absence patterns between similar habitats that currently have and do not have P. colhuapiensis. The goals of the study reported here were to (1) determine the current distribution of A. patagonicus and P. colhuapiensis in the Laguna Blanca wetland system, (2) quantify environmental descriptors (wetland area and other characteristics), and (3) examine how P. colhuapiensis presence and environmental variables may influence the distribution of A. patagonicus.
From January 2003 to March 2006 we surveyed 28 wetlands in the Laguna Blanca wetland system (Fig. 1). Wetlands were identified using topographic maps (Instituto Geográfico Militar, Argentina) and aerial photographs. The perimeter and area of each lagoon was determined using the geographical information system ArcView v. 3.1 (ESRI, Redlands, USA) and a LANDSAT TM image (Path 232 Row 88). For each site we quantified coverage of aquatic vegetation by estimating the percentage of within-wetland aquatic vegetation (emergent Myriophyllum) in three coverage categories: low (< 20%), moderate (20–60%) and high (60–100%).
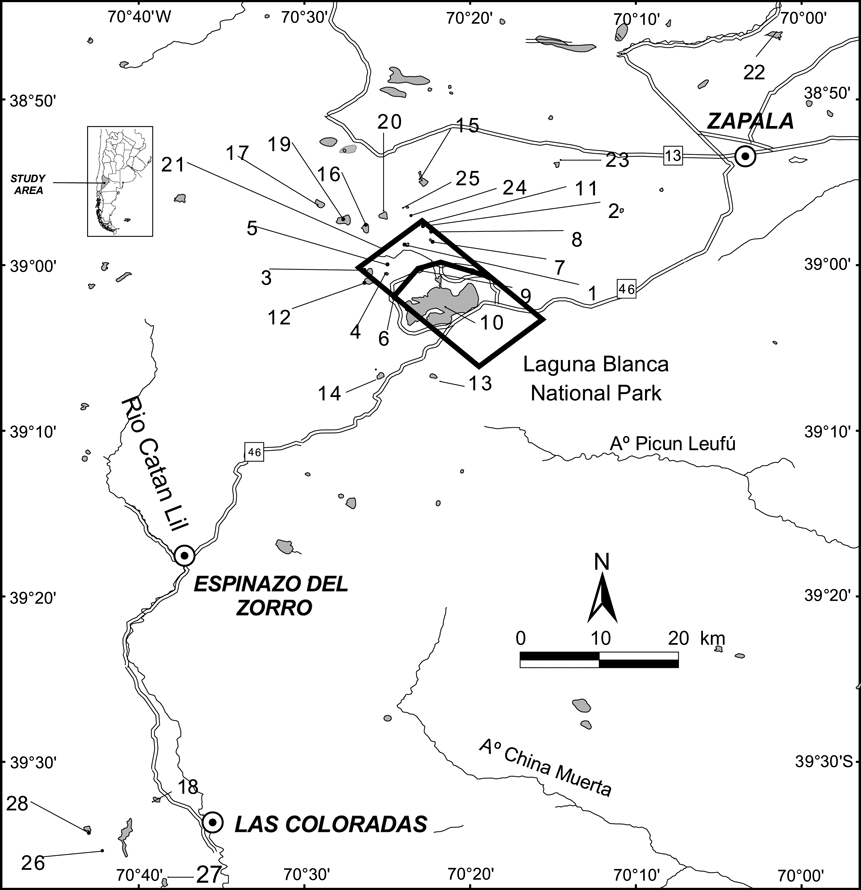
Fig. 1 Laguna Blanca National Park and the surrounding area in Neuquén province. The numbered lagoons are as follows: 1, Antiñir; 2, Antonio; 3, Del Hoyo; 4, Del Molle; 5, Hueso; 6, Batea; 7, Jabón; 8, Montesinos; 9, Verde; 10, Blanca; 11, Blanca Chica; 12, Del Overo; 13, Del Burro; 14, Del Tero; 15, De los Flamencos; 16, Encerrada; 17, El Alamo; 18, La Honda; 19, Panteón; 20, Without name C; 21, Without name B; 22, Solitaria; 23, El Toro; 24, Los Alamitos; 25, Without name A; 26, Los Juncos; 27, Agnata; 28, Colorada. The inset indicates the location of the main figure in Argentina.
To determine presence/absence of A. patagonicus and P. colhuapiensis we used visual encounters at the water's edge, and dip net and funnel trap surveys (Heyer et al., Reference Heyer, Donnelly, McDiarmid, Hayek and Foster1994). Each lagoon was visited on at least two occasions. We took preventive measures to avoid transporting amphibian pathogens between lagoons by following the guidelines of the DAPTF Fieldwork Code of Practice (DAPTF, 1998).
We used the Fisher exact test to examine the relationship between presence/absence of amphibians and fish. Specifically, we tested the null hypothesis that the presence of fish does not affect amphibian presence. Binary logistic regression (Hosmer & Lemeshow, Reference Hosmer and Lemeshow1989), performed with SPSS v. 9.0 (SPSS, Chicago, USA), was used to examine any pattern between the physical attributes of the lagoons and the presence/absence of A. patagonicus and P. colhuapiensis.
A. patagonicus was found in 23 of the 28 lagoons surveyed. We reconfirmed the species' presence in 14 lagoons where it had been previously documented (Fox et al., Reference Fox, Yoshioka, Cuello and Úbeda2005) and in nine new localities, Encerrada, La Honda, Panteón, Unnamed A, B and C, Los Alamitos, Los Juncos and Colorada. The presence of A. patagonicus was associated with lagoons characterized by absence of P. colhuapiensis and high abundance of macrophytes. P. colhuapiensis were present in Blanca and Del Alamo lagoons. Colorada lagoon was stocked with the salmonid Oncorrhynchus mykiss 10 years ago but A. patagonicus are still present. Solitaria, El Toro and Agnata had neither fish nor amphibians.
Twenty-three of the surveyed lagoons that do not have fish are inhabited by A. patagonicus. The null hypothesis that fish presence does not affect amphibian presence/absence was rejected (P = 0.01). A logistic regression model showed that presence of A. patagonicus was positively associated with presence of aquatic vegetation (![]() = 11.21, P < 0.001, R = 0.47) and that presence of P. colhuapiensis was negatively associated with presence of aquatic vegetation (
= 11.21, P < 0.001, R = 0.47) and that presence of P. colhuapiensis was negatively associated with presence of aquatic vegetation (![]() = 4.92, P < 0.0265, R = -0.36).
= 4.92, P < 0.0265, R = -0.36).
Fine sediment shorelines and shallow water characterize Solitaria and Agnata and the absence of complex microhabitats may thus explain the absence of A. patagonicus, which requires coarse sediments and submerged vegetation to breed and develop (Cuello & Perotti, Reference Cuello and Perotti2006). Anthropogenic disturbance over the past 10 years, for water use, may have negatively affected A. patagonicus in the more complex El Toro lagoon, where fish have not been introduced.
A. patagonicus were found in La Colorada where introduced salmonids and abundant macrophytes are present. It is possible that the salmonids may be feeding on benthic invertebrates, which will be commonly available amongst the macrophytes (Macchi et al., Reference Macchi, Cussac, Alonso and Denegri1999).
The percentage of the surveyed lagoons inhabited by A. patagonicus was high (c. 86%) but extirpation of A. patagonicus from Laguna Blanca (the last time it was observed there was in 1986; Iglesias & Pérez, 1998) represents the loss of the largest population. The most severe problem facing the remaining populations is in the many lagoons currently not protected by the National Park. Conservation efforts need to focus on the prevention of fish introductions in the wetland areas on private land.
Many examples have been documented of the negative impacts on native amphibians of fish introduced into historically fishless habitats. The mountain yellow-legged frog Rana muscosa of Kings National Park in California, USA, declined severely as a result of salmonids introduced into previously fish-free lakes (Bradford & Tabatabai, Reference Bradford and Tabatabai1993; Knapp & Matthews, Reference Knapp and Matthews2000) and prevention of this practice along with the removal of non-native fish in several lakes facilitated the recovery of some of the anuran populations (Vredenburg, Reference Vredenburg2004). Fish were introduced to the Laguna Blanca wetland system for sport.
Currently a project to restore A. patagonicus to Laguna Blanca, with participation of Neuquén province, the National Parks Administration and villagers, has begun. In addition, education programmes for villagers and visitors are under development by researchers and rangers, emphasizing the protection of natural Patagonian wetlands as reservoirs of regional biodiversity.
Acknowledgements
We would like to thank the staff of Laguna Blanca National Park and the Game Gardens of Neuquén province for supporting our field work. M. Calvo from the Centro Regional Universitario Bariloche assisted with fieldwork. F. Cruz and G. Dayton reviewed early versions of this article. This study was funded by research grants from the Wildlife Conservation Society (Research Fellowship Program) and a Rufford Small Grant for Nature Conservation to M.E. Cuello and from CONICET to M.G. Perotti. Research was conducted under Permit 081 of National Park and Resolution 885 of Neuquén Province, Argentina.
Biographical sketches
Maria Elena Cuello has been carrying out research on Atelognathus patagonicus in Laguna Blanca National Park and the surrounding area since 2003. María Gabriela Perotti’s research focuses on the evolutionary ecology and conservation biology of South American amphibians. Her recent studies focus on anuran responses to climatic changes, emerging diseases, and environment and species interactions driving amphibian communities. Gustavo José Iglesias is Coordinator of the Biodiversity Information System of the Argentine National Park Administration. This includes a regional database and a geographic information system, with biodiversity information from 36 national protected areas and four natural monuments.





