Introduction
Animal and plant monitoring is the initial tool employed in conservation biology (Marsh and Trenham Reference Marsh and Trenham2008). For species of conservation concern, population monitoring can alert researchers, conservationists, and decision-makers to potential declines in population size (Mace et al. Reference Mace, Collar, Gaston, Hilton-Taylor, Akçakaya, Leader-Williams, Milner-Gulland and Stuart2008, IUCN 2012). Moreover, monitoring populations through time allows for assessment of population status and trends and provides insight into the role that environmental variability (e.g. habitat and food availability, predation, parasites) and human activities may have on ecosystems (Newton Reference Newton1998, Walther et al. Reference Walther, Post, Convey, Menzel, Parmesan, Beebee, Fromentin, Hoegh-Guldberg and Baerlein2002). Data obtained by national or regional surveys are very important to understand the large-scale state of animal populations (i.e. range and abundance), but they are not easily suited for estimating short-term (inter-annual) changes in populations of widely distributed species. In contrast, long-term monitoring of key areas for a species is a commonly used and effective method to identify potential declines in time to take the appropriate measures (e.g. Marsh and Trenham Reference Marsh and Trenham2008, Bretagnolle et al. Reference Bretagnolle, Villers, Denonfoux, Cornulier, Inchausti and Badenhauser2011).
Steppe and farmland birds (hereafter ‘steppe birds’) are the subject of growing conservation concern across Europe, mostly because they have suffered strong declines throughout much of their range over the last decades (Bota et al. Reference Bota, Morales, Mañosa and Camprodon2005, Donald et al. Reference Donald, Sanderson, Burfield, Bierman, Gregory and Waliczky2007). Declines of bird populations in farmlands have been steeper than those of populations of birds associated with other habitats (Gregory et al. Reference Gregory, van Strien, Vorisek, Gmelig Meyling, Noble, Foppen and Gibbons2005, Donald et al. Reference Donald, Sanderson, Burfield and van Bommel2006), a pattern which has raised the conservation profiles of many of these species (Burfield Reference Burfield, Bota, Morales and Mañosa2005). Farmland ecosystems offer critical services for people; thus, political efforts to reconcile conservation and the use of natural resources have focused on providing financial support to farmers (e.g. through LIFE Projects or agri-environment schemes under the Common Agricultural Policy) and on establishing protected areas, e.g. European Special Protection Areas (SPA). Because farmland habitats are extensive in Europe while economic resources for farmland species conservation are limited, resource managers often identify flagship or umbrella species, anticipating respectively that charismatic species would attract public interest and sympathy, or that specific conservation measures undertaken to benefit chosen species will indirectly benefit others that share the same habitat (Simberloff Reference Simberloff1998). Nevertheless, conservation measures for the selected flagship or umbrella species do not necessarily have similar effects on the overall farmland bird community (Concepción et al. Reference Concepción, Díaz, Kleijn, Báldi, Batáry, Clough, Gabriel, Herzog, Holzschuh, Knop, Marshall, Tscharntke and Verhulst2012, Santana et al. Reference Santana, Reino, Stoate, Borralho, Carvalho, Schindler, Moreira, Bugalho, Ribeiro, Santos, Vaz, Morgado, Porto and Beja2014). This has led some researchers to emphasise the need for careful evaluation of the appropriateness of umbrella and flagship species (Caro and Doherty Reference Caro and Doherty1999, Zipkin et al. Reference Zipkin, Royle, Dawson and Bates2010).
The Little Bustard Tetrax tetrax and the Great Bustard Otis tarda are two typical inhabitants of European agro-ecosystems, and the Great Bustard is a particularly charismatic species, owing to its large size (males weigh 10 kg on average) and spectacular displays. Both species co-occur in many areas along their wide distribution range, but have different habitat requirements, niches, and even climatic constraints (Delgado et al. Reference Delgado, Traba and Morales2011, Tarjuelo et al. Reference Tarjuelo, Morales, Traba and Delgado2014, Traba et al. Reference Traba, Morales, Carmona and Delgado2015, Estrada et al. Reference Estrada, Delgado, Arroyo, Traba and Morales2016). The Little and Great Bustards have undergone population declines throughout their European range (Del Hoyo et al. Reference Del Hoyo, Elliot and Sargatal1996), although the latter species has recovered in Spain during recent decades (Palacín and Alonso Reference Palacín and Alonso2008). Due to these population trends, the Little and Great Bustard are considered of high conservation concern, and currently classified globally as ‘Near Threatened’ and ‘Vulnerable’ respectively (BirdLife International 2015). In Spain, which hosts c.50% and 60% of the estimated European populations of Little and Great Bustards, respectively (García de la Morena et al. Reference García de la Morena, Bota, Ponjoan and Morales2006, Palacín and Alonso Reference Palacín and Alonso2008, Alonso and Palacín Reference Alonso and Palacín2010), the two species are listed as ‘Vulnerable’ (Madroño et al. Reference Madroño, González and Atienza2004). Little Bustard populations in Spain are mainly concentrated in the Southern Plateau (46% of the estimated Spanish breeding population) and Extremadura (21%; García de la Morena et al. Reference García de la Morena, Bota, Ponjoan and Morales2006), whereas the largest population of Great Bustard in Spain is located in the Northern Plateau, with 45% of the Spanish population (Alonso et al. Reference Alonso, Palacín and Martín2005a).
In this study, we compare the inter-annual abundance of these two sympatric, closely related bird species, in an important location in Central Spain. We carried out these surveys in the SPA ‘‘Área esteparia del Campo de Calatrava’’, which is representative of Iberian cereal pseudo-steppes and has been highlighted, both internationally (Heath and Evans Reference Heath and Evans2000) and nationally (Traba et al. Reference Traba, García de la Morena, Morales and Suárez2007), for its important steppe bird populations (Martínez Reference Martínez2005). This SPA holds one of the highest average densities of Little Bustard males in Spain and is located in the province that holds the largest population at provincial level in Spain (19% of the estimated total Spanish population; García de la Morena et al. Reference García de la Morena, Bota, Ponjoan and Morales2006). The Great Bustard population in this SPA is similarly important (Gosalvez et al. Reference Gosalvez, Guaman, Segura and Torralvo2002; Alonso et al. Reference Alonso, Palacín and Martín2005a; Arredondo-Acero and López-Jamar Reference Arredondo-Acero and López-Jamar2016). Despite the importance of bustard populations in this region, there has been no analysis of their long-term population trends. In particular, long-term monitoring of sympatric populations of species with different life-history traits would allow determination of species population trends, but also understanding of the relative role of different factors in the conservation of closely related species, and thus allowing for prioritisation of conservation efforts.
Material and Methods
Study area
We carried out this study in Campo de Calatrava (Ciudad Real, central Spain, 38°54’N, 3°55’W, 610 m asl) within the SPA ‘‘Área esteparia del Campo de Calatrava’’ (SPA 157; Figure 1). This area is a slightly undulating agricultural pseudo-steppe dominated by a mosaic of crops, primarily dedicated to dry cereals with interspersed patches of leguminous crops (Vicia spp., Pisum sativum), olive Olea europaea groves, vineyards Vitis vinifera, fallows, pastureland and ploughed fields.
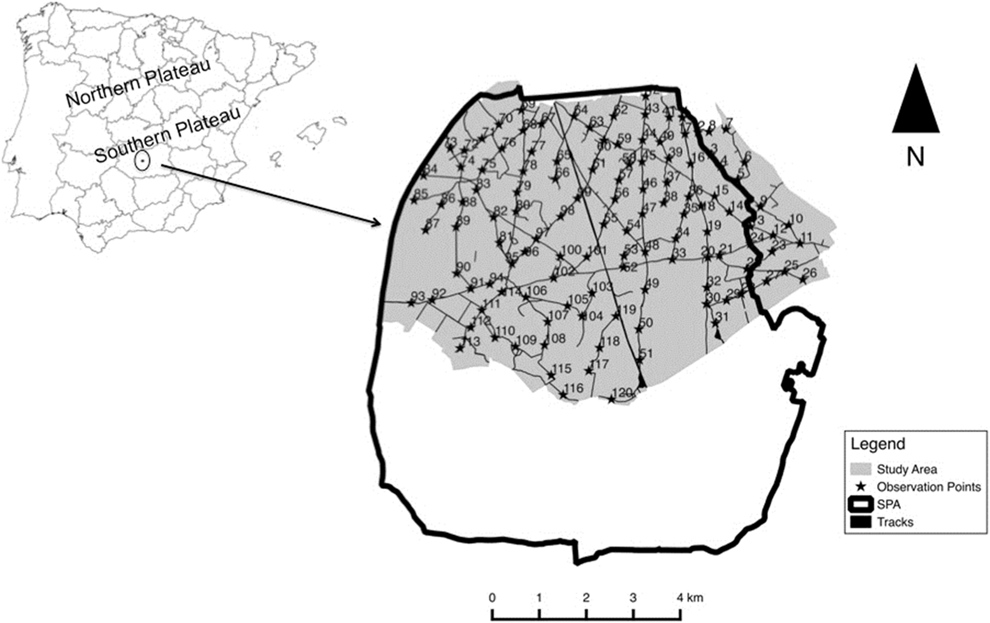
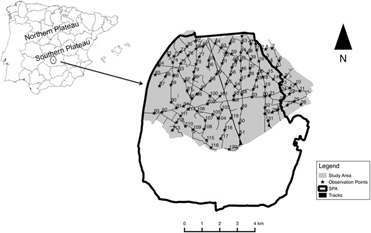
Figure 1. Location of the study area. The inset map shows the limit of our study area (light grey) and the limits of the European Special Protection Area ‘‘Área esteparia del Campo de Calatrava’’ (SPA 157). Black stars (★) and corresponding ID numbers indicate the location of observation points.
Bustard surveys
Little and Great Bustard data were collected annually between 2002 and 2013 in April–early May, coinciding with the mating season peak for both species in our study area (Morales et al. Reference Morales, Casas, García de la Morena, Ponjoan, Calabuig, Martínez-Padilla, García, Mañosa, Viñuela and Bota2014, Tarjuelo et al. Reference Tarjuelo, Morales, Traba and Delgado2014). Surveys were performed using a point count method (Bibby et al. Reference Bibby, Burgess and Hill1992, Díaz-Fernández et al. Reference Díaz-Fernández, Arroyo, Casas, Martinez-Haro and Viñuela2013), and were conducted on consecutive days to cover the entire area, in early morning (from dawn c.3 h later) and late afternoons (last 3 h before dusk), coinciding with the part of the day when birds are more active and detectable (Alonso et al. Reference Alonso, Palacín and Martín2005a, García de la Morena et al. Reference García de la Morena, Bota, Ponjoan and Morales2006). Observation points were spaced every 500–750 m depending on visibility of the surrounding area, and regularly distributed throughout the study area (Figure 1). The average number of observation points surveyed per year was 110 ± 8 (range 91–118). Some observation points were not accessible depending of the year due to track conditions (mostly heavy rain during previous weeks before surveys that made some points inaccessible due to flooding), while others were eliminated due to staff unavailability at the appropriate times. One hundred and six observation points were repeatedly surveyed for at least 10 years, and 57 during the whole study period (12 years). Transects were covered by car at low speed (20 km/h) using local track networks and stopping at the observation points during c.10 min. Little and Great Bustards were detected visually and acoustically from each point and were plotted on a map (1:25,000). Double counting among consecutive points was avoided by cross-checking maps and considering as the same individuals those birds observed in the same place in following days. We calculated bustard abundance indices as the average number of birds detected per observation point (Great Bustard and Little Bustard females and males) each year. Although the employed method is less accurate for counting Little Bustard females due their cryptic plumage and elusive behaviour (Jiguet and Wolff Reference Jiguet and Wolff2000), we have included these results because they are comparable across years as sampling effort is taken into account. Great Bustard flocks were mostly located in the south-east part of our study area (where the two main leks were located), so surveys around these areas were never made in more than one day to avoid double counts.
Statistical analyses
For evaluating the variation in abundance (number of bustards/observation point) per year, we have analysed all data taken from all observation points surveyed between 2002 and 2013. During counts we did not sex Great Bustards in all years, so we decided to include all individuals in the analysis regardless of sex. For the subset of data with sex identification for this species, the trends for both sexes were similar (Figure S1 in the online supplementary material). We explored temporal variations in Great Bustard and Little Bustard female and male abundances using generalized linear mixed models (GLMMs) with a Poisson error distribution and log-link function. We included observation point (to account for the non-independence of data collected in the same observation point in different years) as a random variable and year (a continuous variable) as explanatory variable. We also tested for non-linear relationships with year (2002–2013) by including a quadratic term in the model as an explanatory variable (year2). We used R 3.1.0 (R Core Team 2015) for these analyses.
Results
Between 2002 and 2013 in our study area, Little Bustard abundance showed significantly decreasing non-linear trends in both males (year: χ2 = 4.95, df = 1, P < 0.05, year2: χ2 = 4.94, df = 1, P < 0.05; Figure 2) and females (year: χ2 = 55.67, df = 1, P < 0.001, year2: χ2 = 55.50, df = 1, P < 0.001; Figure 2), while Great Bustards showed a significant increasing non-linear trend (year: χ2 = 4.73, df = 1, P < 0.05, year2: χ2 = 4.62, df = 1, P < 0.05; Figure 2). The number of Little Bustard breeding males and females suffered a decline of c.56% and 55% respectively (from an estimated total of 176 males and 29 females in 2002 to 77 males and 13 females in 2013), whereas the Great Bustard population experienced a remarkable increase of 1,800% (from five individuals in 2002 to 90 individuals in 2013). The average annual decrease rate for Little Bustards between 2002 and 2013 was 5.3%, and the average annual growth rate for Great Bustard was 163.6%.
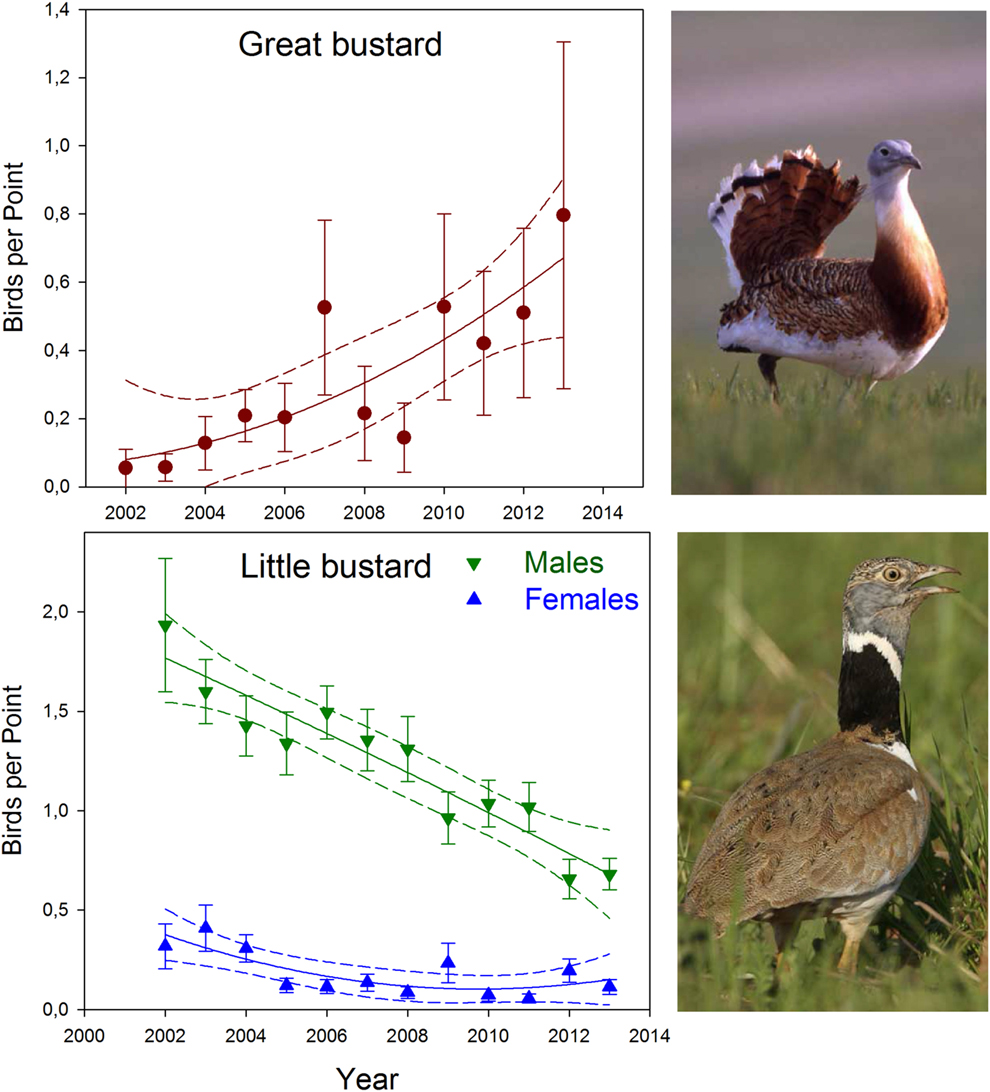
Figure 2. Great Bustard and Little Bustard average abundance (± SE) by year between 2002-2013. Solid lines show the population trend and dashed lines show the 95% confidence intervals.
Discussion
Little and Great Bustards showed significant opposite population trends over a 12-year period in our study area. As far as we know, this is the longest continuous annual survey of co-occurring bustard breeding populations in Spain. In particular, the Little Bustard population located in Campo de Calatrava SPA showed a significant decline in both males and females, while the Great Bustard population has markedly increased. These results for the Spanish Great Bustard population are consistent with those reported at different geographical scales and temporal periods in the last two decades, that have shown an overall, albeit irregular increase, depending on the area: populations located in Madrid have decreased recently (Palacín and Alonso Reference Palacín and Alonso2018) and although the Extremadura population seems to have also suffered a decrease, recent trends for Badajoz province between 1988 and 2011, show than this population is holding stable (Sanchez and Garcia-Baquero Reference Sanchez and Garcia-Baquero2012). By contrast, Little Bustard populations have suffered a generalised sharp decline across all their south-west European range (Alonso et al. Reference Alonso, Palacín and Martín2005a, SEO/BirdLife 2016, Silva et al. Reference Silva, Correia, Alonso, Martins, D’Amico, Delgado, Sampaio, Godinho and Moreira2018; Table 1). However, this synopsis also highlights the lack of accurate information for Great Bustards in Castilla-La Mancha. Campo de Calatrava SPA is considered one the best locations for Little Bustards in the region, showing one of the highest average densities of Little Bustard males in Spain (García de la Morena et al. Reference García de la Morena, Bota, Ponjoan and Morales2006). Therefore, the recent steep decline of this population in our study area appears similar to the region-wide situation of Little Bustard populations for Castilla-La Mancha, which would then represent an important decrease for Spain as a whole (Table 1).
Table 1. Recent population trends for Little and Great Bustard in their most important range in south-west Europe. Some examples of regional population trends for both species (more information available in Alonso et al. Reference Alonso, Palancìn and Martìn2003, Reference Alonso, Palacín and Martín2005a and Garcia de la Morena et al. Reference García de la Morena, Bota, Mañosa and Morales2017).

1 Data not available for these regions. *Species not present in this region
As regards the Great Bustard, our results add another piece of evidence for this species’ current recovery in Iberia, presumably thanks to a combination of conservation efforts specifically focused on the Great Bustard implemented since the 1990s, along with a hunting ban in Spain and Portugal in the 1980s (Alonso et al. Reference Alonso, Palacín and Martín2005a, Martín et al. Reference Martín, Martín, Bautista and Martín2012, Alonso Reference Alonso2014, SEO/BirdLife 2016). For example, in Castilla y León, the region with the largest population of Great Bustards in the world, which includes 47% of the Spanish population (Alonso et al. Reference Alonso, Palacín and Martín2005a), the Great Bustard population has increased by 34%, favoured by an increase in the extent of unirrigated legume crops (Martín et al. Reference Martín, Martín, Bautista and Martín2012). Nevertheless, these crop changes only partly explain the local increases in Great Bustard populations, because not all Great Bustard areas have been subject to these crop changes, and Great Bustards have a strong conspecific attraction and site fidelity (Alonso et al. Reference Alonso, Alonso and Martín2004). Indeed, the species shows a tendency to concentrate in occupied areas with good habitat conditions, and it will decrease or even suffer local extinctions in marginal populations (Palacín et al. Reference Palacín, Alonso, Martin, Martí and Del Moral2003, Pinto et al. Reference Pinto, Rocha and Moreira2005). In our study area, the increase in Great Bustard numbers appears to be due to the combination of the general increase in the Great Bustard population in Ciudad Real province (Table 1), but also by the displacement of individuals from nearby marginal locations (see Arredondo-Acero and López-Jamar Reference Arredondo-Acero and López-Jamar2016). In particular, an increase in the construction of man-made structures (e.g. Ciudad Real airport) at the southern edge of Campo de Calatrava SPA and associated human-related disturbances could have induced the displacement of birds to less disturbed areas (López-Jamar et al. Reference López-Jamar, Casas, Díaz and Morales2011), and would explain the large difference in the rate of increase between our study area and the whole population of Ciudad Real province (Table 1). In this sense, the spatial distribution of Great Bustards in our study area agrees with the general trend reported for the whole country, with an overall increasing number of birds, but concentrated in an increaseingly reduced distribution area with optimal conditions for the species, while marginal smaller populations decline or even disappear (Alonso et al. Reference Alonso, Palancìn and Martìn2003, Reference Alonso, Palacín and Martín2005a,Reference Alonso, Martín, Palacín, Martín and Magañab).
Little and Great Bustard have different habitat requirements and ecological differences, which would explain the different trends found between these two species. Fallow fields are positively selected for nesting by these two species (Magaña et al. Reference Magaña, Alonso, Martín, Bautista and Martín2010, Morales et al. Reference Morales, Traba, Delgado and García de la Morena2013). However, agricultural intensification and habitat change have reduced the area of this important habitat, so that cereal fields are the main alternative habitat for nesting (Magaña et al. Reference Magaña, Alonso, Martín, Bautista and Martín2010, Lapiedra et al. Reference Lapiedra, Ponjoan, Gamero, Bota and Mañosa2011). Given that Little Bustards nest later than Great Bustards, they may suffer more nest losses at crop harvest time. Cereals could thus act as an ecological trap for Little Bustard, especially in intensive cereal-dominated landscapes (Lapiedra et al. Reference Lapiedra, Ponjoan, Gamero, Bota and Mañosa2011), as it has been considered for species with similar nesting habitat requirements (Casas and Viñuela Reference Casas and Viñuela2010). In addition, the Great Bustard, which is 10 times larger than the Little Bustard, can feed and shelter in habitats where herbaceous vegetation is too tall and dense for the Little Bustard (which seems to prefer vegetation shorter than 30 cm; Morales et al. Reference Morales, García de la Morena, Delgado and Traba2008a). Therefore, landscape homogenisation with intensive, densely sown winter cereals (already too tall in the breeding season) might be harmful for the Little Bustard, but not necessarily so for the Great Bustard. Finally, some evidence indicates that Great Bustard may be a more herbivorous specialist than Little Bustard. Lane et al. (Reference Lane, Alonso, Alonso and Naveso1999) indicate that Great Bustards eat cereal leaves and seeds, which are a superabundant resource (their relatively larger intestinal caecum may allow them to more efficiently process fibre and cellulose). However, the few dietary studies available for Little Bustard (Jiguet Reference Jiguet2002, Bravo et al. Reference Bravo, Cuscó, Morales and Mañosa2017), the latter with data from several very different and distant wintering localities) show that there is no sign of cultivated graminae in faeces, neither green parts nor seeds. Furthermore, even in cereal farmland, Little Bustards show a marked preference for legumes (both cultivated and wild) and other wild plants, a landscape element clearly selected by lekking males and by breeding females (Martínez Reference Martínez1998, Faria et al. Reference Faria, Rabaça and Morales2012), which are much scarcer resources in current agrarian landscapes (Bravo et al. Reference Bravo, Cuscó, Morales and Mañosa2017). In this sense, an adequate management of alfalfa fields promoted by EU agri-environmental aids has been key to recovering an almost extinct Little Bustard population in France for which this is a crucial breeding habitat (Bretagnolle et al. Reference Bretagnolle, Villers, Denonfoux, Cornulier, Inchausti and Badenhauser2011). However, leguminous crops were scarce in our study area (Casas and Viñuela Reference Casas and Viñuela2010), almost exclusively peas, which are harvested even earlier than cereals, so they could also be a trap for breeding females (like traditionally managed alfalfas in France; Bretagnolle et al. Reference Bretagnolle, Villers, Denonfoux, Cornulier, Inchausti and Badenhauser2011). It could be an ephemeral food resource too, absent in full summer, when green vegetation in Mediterranean areas is more scarce. Furthermore, in the only two available studies on chick diet, to our knowledge, Little Bustard chicks feed almost exclusively on arthropods (Jiguet Reference Jiguet2002), while young Great Bustard diet includes about 50% of plant material (Bravo et al. Reference Bravo, Ponce, Palacín and Alonso2012), so perhaps Little Bustards are more affected by decreases in arthropod abundance in intensively managed agricultural systems.
Because large or charismatic flagship species can attract more public attention and resources than others, they are often considered also as umbrella species, even without evaluating the coverage they provide for other species (Simberloff Reference Simberloff1998, Andelman and Fagan Reference Andelman and Fagan2000). The Great Bustard has frequently been considered the flagship of Iberian (and Eurasian) farmland steppe ecosystems (Morales and Traba Reference Morales and Traba2016), although it has in practice been treated as an umbrella species, whose protection may have benefitted steppe bird populations. However, because this species has different habitat requirements to other steppe bird species, habitat management actions undertaken to benefit Great Bustard populations may not benefit other species, as is the case for the Little Bustard (Tarjuelo et al. Reference Tarjuelo, Morales, Traba and Delgado2014), probably due to their different response to agriculture intensification. For example the spread of dry alfalfa (promoted by agri-environmental schemes) that has taken place in Castilla y León to benefit Great Bustard populations (Martin et al. Reference Martín, Martín, Bautista and Martín2012) would not necessarily benefit Little Bustards, because they breed later than Great Bustard and alfalfa cutting schemes (particularly date of first mowing) of these alfalfas are adapted to Great Bustard (slightly delayed with respect to normal management), but no so much to Little Bustard. Besides, although interspecific competition between these two closely related species may not be so important at large scale, it may be a current additional risk for Little Bustards due to the increasing populations of Great Bustards in areas where the two species are forced to occupy cereal crops, including our study area (Tarjuelo et al. Reference Tarjuelo, Morales, Arroyo, Mañosa, Bota, Casas and Traba2017a,Reference Tarjuelo, Traba, Morales and Morrisb). Concretely, Great Bustard would displace Little Bustard to their “primary” habitat, fallows and pastures (Tarjuelo et al. Reference Tarjuelo, Morales, Arroyo, Mañosa, Bota, Casas and Traba2017a,Reference Tarjuelo, Traba, Morales and Morrisb). Given that these habitats are very scarce, they could have problems finding enough resources at population level (food or nesting sites, for example). Specific conservation actions or ’business as usual’ in agriculture would have been enough to allow the recovery of Great Bustard after the hunting ban, but not for the Little Bustard. Because of this effect, selecting several umbrella species with different habitat requirements would be a more appropriate choice for advancing conservation of agro-steppe lands than setting target population sizes for the Great Bustard alone.
Annual monitoring of local populations (selected by virtue of numerical importance, habitat preferences, and distributional range) is an important conservation tool for widely distributed species. This approach is especially valuable in highly managed landscapes that are subject to intense local changes in land use and human disturbance rates. In Spain, the common bird monitoring programme hosted by SEO/BirdLife (SACRE) provides the best information of population trends for common bird species that are widely distributed throughout Spain (SEO/BirdLife 2016). However, this monitoring programme does not permit estimation of the total number of individuals of a particular species in a particular location. On the other hand, nationwide surveys of Little Bustard populations are carried out in Spain roughly every 10 years, which are useful for estimating global population abundance but not for detecting short-term population trends. In the case of the Great Bustard, most surveys are usually framed by different regional or provincial monitoring programmes, which vary temporally with conservation priorities and funding. These differences contribute to unequal and asynchronous survey efforts among provinces or regions, which may reduce the accuracy of these large-scale monitoring efforts (Alonso et al. Reference Alonso, Palacín and Martín2005a).
Our results illustrate the utility of local, long-term population surveys, highlighting the delicate state of conservation of the Spanish Little Bustard populations. The trends for Little and Great Bustards are strongly divergent in this shared habitat, but these local trends accord with the regional trajectories. Because of the differences in species trends, we recommend that monitoring efforts must be more intensive and frequent for Little Bustards than for Great Bustard populations. In particular, the current regional monitoring programmes would be sufficient for the Great Bustard populations (e.g. every 10 years in Castilla y León; Martín et al. Reference Martín, Martín, Bautista and Martín2012). However, because the Little Bustard population has declined so much in the last 15 years, and because the Spanish population of Little Bustards is important at the European level (García de la Morena et al. Reference García de la Morena, Bota, Ponjoan and Morales2006), more survey efforts are needed for this species. For example, it would be useful to conduct annual surveys at each of several important locations for the species throughout its breeding range in Spain. The number of survey locations per region could be set according to the population importance of Little Bustard in each region (e.g. 2–3 locations in provinces with larger populations versus only 1 location in provinces with smaller populations). This survey strategy would provide a good set of data for estimating population abundance annually, as well as information on population trends at large-scales, where changes in land-use, agricultural practices, and other human activities (i.e. hunting and leisure activities) could affect Little Bustard and other species’ populations (Casas et al. Reference Casas, Mougeot, Viñuela and Bretagnolle2009, Tarjuelo et al. Reference Tarjuelo, Barja, Morales, Traba, Benítez-López, Casas, Arroyo, Delgado and Mougeot2015, Casas et al. Reference Casas, Benítez-López, Tarjuelo, Barja, Viñuela, García, Morales and Mougeot2016). This shift in survey effort and resources appears warranted now that the Little Bustard is markedly declining while the less imperilled Great Bustard is either stable or overall increasing, in their south-west European range, although in highly threatened population as the one in Madrid region a strong recent population decline has been recorded recently (Palacín and Alonso Reference Palacín and Alonso2018).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270918000254
Acknowledgements
We are very thankful to all people that help us with fieldwork. Fidel Sánchez-Campos helped us with GIS data. We also thank Rafael Palomo for his excellent pictures (Figure 2). This work was supported by the Ministerio de Ciencia y Tecnología (MCYT-REN2003-07851/GLO, CGL2004-02568/BOS, CGL2007-66322/BOS and CGL2008-04282/BOS) and the Project ‘‘Steppeahead’’ funded by Fundación General del Consejo Superior de Investigaciones Científicas from Spain (FGCSIC) and Banco Santander. FC was supported by the Andalucía Talent Hub Program launched by the Andalusian Knowledge Agency, cofounded by the European Union’s Seventh Framework Program, Marie Skłodowska-Curie actions (COFUND–Grant Agreement n° 291780) and the Ministry of Economy, Innovation, Science and Employment of the Junta de Andalucía.