Introduction
Effective conservation action depends fundamentally on quantitative assessments of the size, distribution and temporal trends of populations. Assessment of population trends are especially important for long-lived species with low annual reproductive rates, as such species may be less capable of rebounding quickly from reductions in population size caused by natural or anthropogenic factors (Heinsohn et al. Reference Heinsohn, Zeriga, Murphy, Igag, Legge and Mack2009, Lotze et al. Reference Lotze, Coll, Magera, Ward-Paige and Airoldi2011). Furthermore, high adult survival in such species may mask the long-term consequences of low reproductive rates such as an ageing demographic structure with an increasing proportion of non-breeders (Lee et al. Reference Lee, Reid and Beissinger2017). Such populations run the very real risk of rapid crashes when large proportions of the population reach reproductive senescence. In social species, the propensity for such crashes can be compounded by Allee effects, in which population growth becomes negative when population size drops below a threshold at which social groups no longer function (Courchamp et al. Reference Courchamp, Berec and Gascoigne2008, Hutchings Reference Hutchings2015).
The parrots and cockatoos (Order Psittaciformes, hereafter ‘parrots’) are among the longest-lived group of birds and have generally low annual rates of reproduction (Young et al. Reference Young, Hobson, Lackey and Wright2012, Toft and Wright Reference Toft and Wright2015). They typically have a complex fission-fusion social organisation in which long-term pair bonds are maintained within feeding flocks that in turn merge at communal night roosts (Toft and Wright Reference Toft and Wright2015, Bradbury and Balsby Reference Bradbury and Balsby2016). The parrots are also the most endangered large order of birds, with 112 of 398 extant species (28%) classified on the 2016 IUCN Red List as ‘Vulnerable’, ‘Endangered’ or ‘Critically Endangered’, and an additional 60 species (15%) listed as ‘Near Threatened’ (BirdLife International 2016b). Although the causes of endangerment vary among species, two predominate: loss of suitable habitat and capture for the pet trade (Snyder et al. Reference Snyder, McGowan, Gilardi and Grajal2000, Wright et al. Reference Wright, Toft, Enkerlin-Hoeflich, Gonzalez-Elizondo, Albornoz, Rodríguez-Ferraro, Rojas-Suárez, Sanz, Trujillo, Beissinger, Berovides, Gálvez, Brice, Joyner, Eberhard, Gilardi, Koenig, Stoleson, Martuscelli, Meyers, Renton, Rodríguez, Sosa-Asanza, Vilella and Wiley2001, Pain et al. Reference Pain, Martins, Boussekey, Diaz, Downs, Ekstrom, Garnett, Gilardi, McNiven, Primot, Rouys, Saoumoe, Symes, Tamungang, Theuerkauf, Villafuerte, Verfailles, Widmann and Widmann2006, Olah et al. Reference Olah, Butchart, Symes, Guzman, Cunningham, Brightsmith and Heinsohn2016, Berkunsky et al. Reference Berkunsky, Quillfeldt, Brightsmith, Abbud, Aguilar, Aleman-Zelaya, Aramburu, Ariash, McNab, Balsby, Barberena, Beissinger, Rosales, Berg, Bianchi, Blanco, Bodrati, Bonilla-Ruz, Botero-Delgadillo, Canavelli, Caparroz, Cepeda, Chassot, Cinta-Magallon, Cockle, Daniele, de Araujo, de Barbosa, de Moura, Del Castillo, Diaz, Diaz-Luque, Douglas, Rodriguez, Garcia-Anleu, Gilardi, Grilli, Guix, Hernandez, Hernandez-Munoz, Hiraldo, Horstman, Portillo, Isacch, Jimenez, Joynerak, Juarez, Kacoliris, Kanaan, Klemann, Latta, Lee, Lesterhuis, Lezama-Lopez, Lugarini, Marateo, Marinelli, Martinez, McReynolds, Urbinaat, Monge-Arias, Monterrubio-Rico, Nunes, Nunes, Olaciregui, Ortega-Arguelles, Pacifico, Pagano, Politi, Ponce-Santizo, Reyes, Prestes, Presti, Renton, Reyes-Macedo, Ringler, Rivera, Rodriguez-Ferraro, Rojas-Valverde, Rojas-Llanos, Rubio-Rocha, Saidenberg, Salinas-Melgoza, Sanz, Schaefer, Scherer-Neto, Seixas, Serafini, Silveira, Sipinski, Somenzari, Susanibar, Tella, Torres-Sovero, Trofino-Falasco, Vargas-Rodriguez, Vazquez-Reyes, White, Williams, Zarza and Masello2017). The pet trade may have a particularly pernicious effect on populations as poaching often targets nestlings, leaving a standing adult population that may appear healthy but suffers from low recruitment of young birds. There are many anecdotes of rapid declines of parrot populations that may be caused entirely or in part by ageing population structures driven by the pet trade (Snyder et al. Reference Snyder, McGowan, Gilardi and Grajal2000, BirdLife International 2016a), but a lack of repeated and quantitative census data for most species makes it difficult to determine how widespread such crashes are.
The Yellow-naped Amazon Amazona auropalliata is a widespread parrot species that inhabits the tropical dry forest habitat that extends along the Pacific slope of Mesoamerica from southern Mexico to northern Costa Rica (Forshaw Reference Forshaw2006). It is currently classified as ‘Endangered’ on the IUCN Red List and is thought to be declining across all of its range (BirdLife International 2017), but relatively few systematic surveys of population numbers have been conducted. Costa Rica populations have been the subject of a long-term study of geographic variation in vocalisations that have included surveys of night roosts in 1994, 2005 and 2016 (Wright Reference Wright1996, Wright et al. Reference Wright, Dahlin and Salinas-Melgoza2008). Surveys in Nicaragua conducted in 1994, 1999 and 2004 found continuous declines in numbers sighted at point counts over this 10 year span (Lezama-López Reference Lezama-López, Rich, Arizmendi, Demarest and Thompson2009). Reports from elsewhere in its range note declining populations, loss of key habitat, and large numbers of birds exported for the pet trade (Wiedenfeld Reference Wiedenfeld1993, Grijalva Reference Grijalva2008, BirdLife International 2016b). Costa Rica is generally held to be a stronghold of the species, but small-scale studies of nesting success have reported high levels of nest poaching (Wright et al. Reference Wright, Toft, Enkerlin-Hoeflich, Gonzalez-Elizondo, Albornoz, Rodríguez-Ferraro, Rojas-Suárez, Sanz, Trujillo, Beissinger, Berovides, Gálvez, Brice, Joyner, Eberhard, Gilardi, Koenig, Stoleson, Martuscelli, Meyers, Renton, Rodríguez, Sosa-Asanza, Vilella and Wiley2001, BirdLife International 2017, Dahlin et al. Reference Dahlin, Chelsea, Rising and Wright2018) and this species is commonly found as pets in Costa Rican households (Drews Reference Drews, Salem and Rowan2003). Overall, current estimates of the total population of the Yellow-naped Amazon vary widely from 10,000 to 50,000 individuals, reflecting considerable uncertainty in the status of different populations (BirdLife International 2017).
Costa Rica and Nicaragua in particular represent an interesting contrast in land-use regimes and approaches to conservation that could potentially impact parrot populations. Over the last 40 years, Costa Rica has emphasised protection and regulation of large swathes of its national territory in a system of “Áreas de Conservación” that include national parks, wildlife refuges and biological reserves that encompass many different ecosystems and enjoy some degree of protection from over-exploitation (Janzen and Hallwachs Reference Janzen, Hallwachs and Kappelle2016). A much smaller portion of Nicaragua’s territory is under protected status, and a large proportion of what is protected is owned by private landowners who agree to dedicate part of their holdings to conservation and restoration of native habitat, a system known as “Reservas Silvestres Privadas” (Anonymous 2013a). Outside of these protected areas, large parcels of Nicaragua’s Pacific slope are devoted to high-intensity commercial or small-scale subsistence agriculture. The effects of the differences between these two countries in land-use and conservation regimes on the health of Yellow-naped Amazon populations are currently unknown.
In this study we report the results of a systematic census conducted in June and July 2016 of population numbers and group sizes at night roosts of the Yellow-naped Amazon in Costa Rica and Nicaragua. Our comparison of populations in the contiguous range of this species on the Pacific slope of the two countries allows us to contrast trends between two neighbouring countries with different land-use and conservation regimes, while measurement of groups sizes provides a rough estimate of reproductive success. We also compare these results to those from a 2005 survey in Costa Rica, providing a window on temporal trends within this country between 2005 and 2016.
Methods
Study area
We conducted surveys of roosts in the Pacific lowlands of northwestern Costa Rica and southwestern Nicaragua from 1994 to 2016 (Figure 1). This region lies in the tropical dry forest biome and is characterised by strong seasonality, with a rainy season running from June to December, and a dry season with little rainfall from January to April. It lies at the southern end of the range of the Yellow-naped Amazon, which runs from north-western Costa Rica to south-western Mexico (BirdLife International 2017). Broad scale surveys were conducted in Costa Rica in 2005 (6–23 June) and 2016 (3-29 June) and in Nicaragua in 2016 (24 June–10 July). This period of the year coincides with the post-fledging period when young can be observed in close association with their parents at roosts and feeding sites (T. Wright, C. Dahlin & M. Lezama unpubl. data) as seen in other amazon parrots (Salinas-Melgoza and Renton Reference Salinas-Melgoza and Renton2007). Additional smaller-scale surveys were conducted in Costa Rica in April and May 1994 and in Nicaragua in May 2008 and June 2009, 2011 and 2014. Potential roost sites for surveys were identified via previous field studies (Wright Reference Wright1996, Lezama et al. Reference Lezama, Vilchez, Mayorga and Castellón2004, Wright et al. Reference Wright, Dahlin and Salinas-Melgoza2008), consultation with local experts and residents, and in 2016, by reports of sightings registered on the Cornell Lab of Ornithology’s eBird website (www.ebird.org).
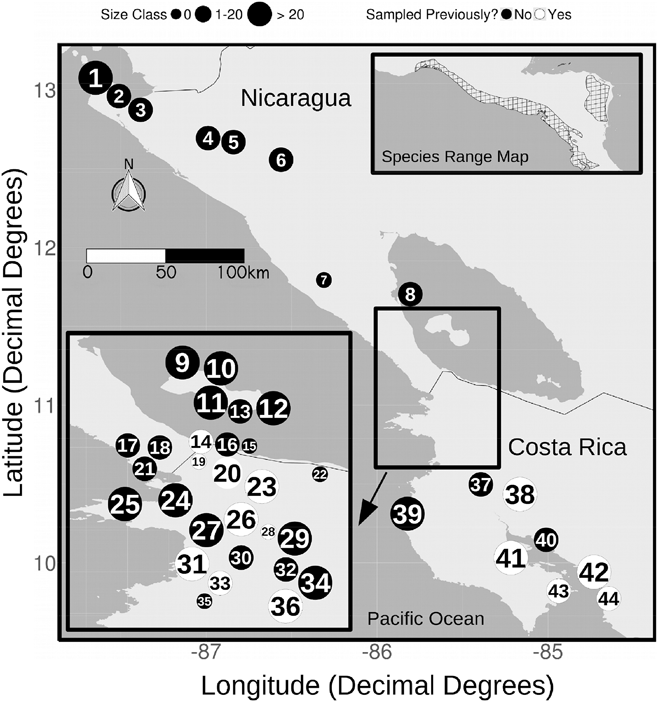
Figure 1. A map of roost sites surveyed in Costa Rica and Nicaragua. Sites are labelled with numbers corresponding to the numbers in Table 1. The size of the site label represents the relative number of birds observed at that roost (small = 0 birds, medium = 1–20 birds, large = > 20 birds). Filled circles were only surveyed in 2016, open circles were also surveyed in earlier years. The species range polygon is provided by BirdLifeInternational and NatureServe (2015).
Roost counts and reproductive success
Roost counts were conducted in either the evening, as birds arrived at the roost, or in the early morning when birds departed from the roost, with only one count conducted per roost. We counted birds in the evening from 17h00 until nightfall at approximately 18h30 and in the morning from first light at approximately 05h15 until 06h30. Yellow-naped Amazons are diurnal and rarely fly in the dark (T. Wright, C. Dahlin & M. Lezama unpubl. data), so these two time-periods represented the best times to estimate the number of parrots arriving at or departing from night roosts (Cougill and Marsden Reference Cougill and Marsden2004, Matuzak and Brightsmith Reference Matuzak and Brightsmith2007). Cougill and Marsden (Reference Cougill and Marsden2004) conducted morning and evening roost counts at a single roost of the Red-tailed Amazon Amazona brasilensis in Brazil and observed no systematic bias in the number of birds observed in the morning versus the evening; for that reason we include data from both types of counts in our study.
Roost counts were conducted by one or two observers. The topography of different roost sites required different positioning of observers as lines of sight varied among sites; generally when the local landscape permitted, two observers were positioned on opposite sides of the roost allowing for maximum arc of sight. At roosts where it was only possible for birds to fly from certain directions (i.e. in mangrove patches bordered by the ocean) one observer was positioned with a full line of sight over the birds’ flight path. Birds were counted as they came in to roost based on visual observations, and the direction from which they flew recorded so that any double counting of birds could be corrected when counts from multiple observers were combined. For some sites at which formal counts were not conducted or were not possible due to logistical or geographic constraints, we estimated the size of the population based on the birds seen and heard in the area. Although we believe count data give a more accurate assessment of roost size, the estimate data are still valuable for helping to generate an overall picture of population size across the region surveyed. We differentiate between the two types of data in Table 1.
Table 1. Population counts, estimates and reproductive success at sites in Costa Rica and Nicaragua.
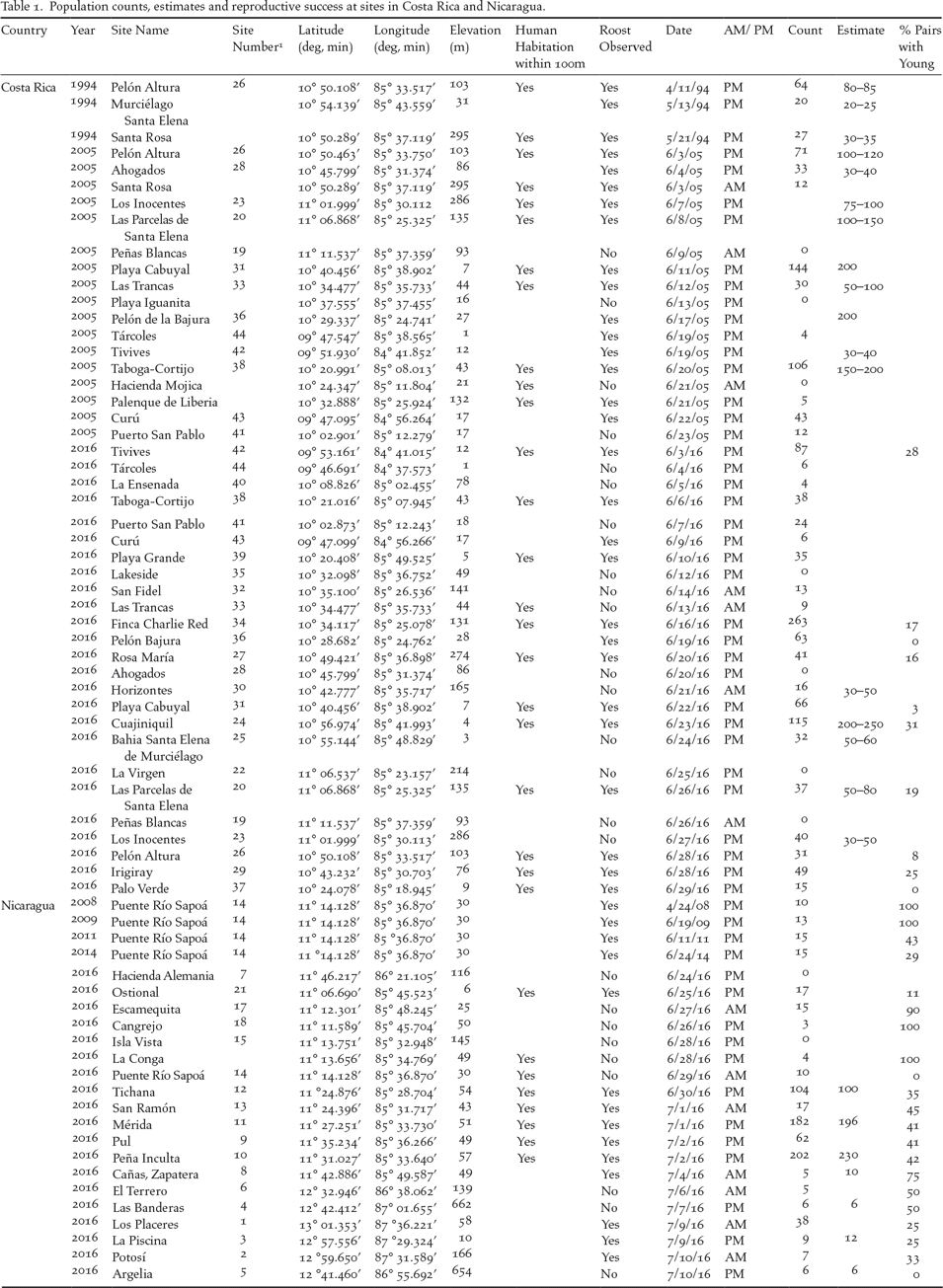
1 Site numbers correspond to numbers on the map in Figure 1 and ascend from north to south.
To estimate reproductive success, we recorded the size of groups of birds as they flew into the roost. Our own observations (T. Wright, C. Dahlin & M. Lezama unpubl. data) and other studies of amazon parrots (Matuzak and Brightsmith Reference Matuzak and Brightsmith2007, Salinas-Melgoza and Renton Reference Salinas-Melgoza and Renton2007) suggest that groups flying in close proximity, particularly in the post-fledging period, often represent mated pairs accompanied by their recently fledged young. In 2016, we recorded the size of groups and used those data to estimate the proportion of groups that consisted of reproductively successful pairs with recently fledged offspring (e.g. groups of > 2 individuals). This approach does depend critically on the assumption that any groups consisting of three or more birds represent a successful reproduction event. Any groups of three or more that consist of multiple adult pairs or of unaffiliated juveniles would inflate this estimate of reproductive success.
Roost site characteristics
In addition to counts, we recorded descriptive data for each roost site. Location (latitude and longitude) and elevation were recorded for most sites with a handheld GPS unit; in a few cases location was calculated post hoc using Google Earth (vers. 7.1.7.2602) and elevation with the website Geoplaner (www.geoplaner.com). In 2016, we also classified the predominant type of roost substrate as either mangrove or mixed deciduous forest, and recorded whether the roost was within 100 m of human habitation as a measure of tolerance of anthropogenic disturbance.
Data analysis
Roost counts for roosts surveyed in 2016 were summed over the entire survey area to obtain an estimate for the entire population, and also within Costa Rica and Nicaragua separately to obtain a minimum population size for each country. We tested for a difference in the mean roost sizes between the two countries using a t-test. For roosts surveyed both in 2016 and earlier years we graphed the counts by year and tested for a difference between sites counted in both 2005 and 2016 using a paired t-test to estimate broad population trends over the 11-year period. We excluded from this analysis sites at which no birds were counted in either the 2005 or 2016 surveys. We calculated the proportion of pairs with fledglings as an estimate of reproductive success and compared this estimate between Costa Rica and Nicaragua using a t-test. We tested for differences in roost characteristics between Costa Rica and Nicaragua using t-tests or Fisher’s exact tests as appropriate. We tested for associations between roost size and roost characteristics using t-tests or linear regressions. All proportions were calculated in Excel (vers 14.6.8) and graphs made and statistical tests conducted in JMP (vers 10.0.2). The alpha level for significance for all tests is P < 0.05, and all means are reported ± SD.
Results
Roost counts: Regional trends
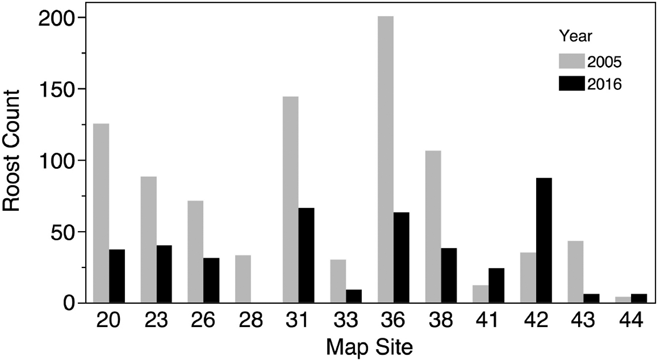
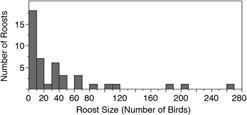
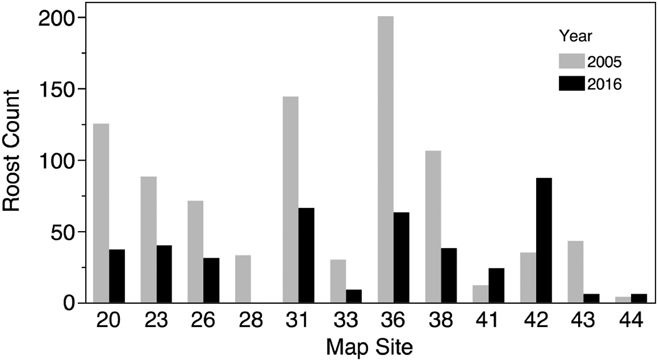
We conducted roost counts at 44 sites in Costa Rica and Nicaragua over 37 days in 2016 and observed birds roosting at 23 of these sites (Table 1, Figure 1). The total number of individuals counted in 2016 was 1682 birds, the maximum roost count was 263 birds and the mean per site was 38.2 ± 56.7 birds. The distribution of roost sizes was highly skewed, however, as the median roost size was 15.5 birds, 25 roosts (57%) had 20 birds or less, and only 9 roosts (20%) had 50 or more birds counted (Figure 2).
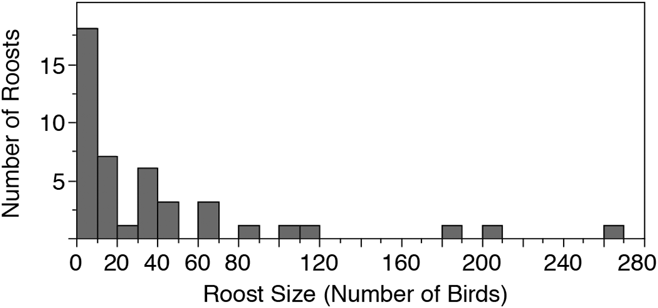
Figure 2. A histogram representing the relative distribution of roost counts in Costa Rica and Nicaragua in 2016. 80% of 44 roost sites observed had 50 or fewer birds.
Roost counts: Trends by country
In Costa Rica we conducted roost counts at 25 sites in 2016, and observed birds roosting at 13 of these sites. At 8 of the other 12 sites birds were counted but roosting was not confirmed (i.e. the birds departed the area before nightfall), while at the remaining four sites no birds were observed during the count. The mean number counted at all sites was 40.0 ± 54.8 birds, while the mean counted at confirmed night roosts was 60.4 ± 65.9 birds. Only five roosts had roost counts of more than 50 birds; these roosts were dispersed across the range of this species in Costa Rica, with three located in or near coastal mangrove forests and two in mixed-deciduous forest in agricultural areas (Figure 1). The maximum roost count was 263 birds and the total number of birds counted across all sites in Costa Rica was 990 birds.
We conducted counts at three sites in 1994 and counts or estimates at 17 sites in 2005 in Costa Rica (Table 1, Figure 1). The mean roost size at all sites in 1994 was 37 ± 24.6 birds, while the mean of all sites counted in 2005 was 38 ± 46.3. We conducted counts or estimates at 12 sites in 2005 that were also included in the 2016 survey. Nine of the 12 roosts showed reductions in population numbers from 2005 to 2016 (Figure 3), and the mean roost size decreased from a mean of 74 ± 59.9 birds in 2005 to 33.9 ± 27.3 in 2016, a mean decline of 54% in the 11 years separating the two counts. This decline was statistically significant (paired t-test, df = 11, t-ratio = -2.8, P < 0.02) and remained significant when only the 6 roosts that had actual counts in both surveys were considered (paired t-test, df = 5, t-ratio = -5.1, P < 0.004).
In Nicaragua we conducted roost counts at 19 sites in 2016, and observed birds roosting at 10 of these sites (Table 1, Figure 1). At seven of the other nine sites birds were counted but roosting was not confirmed, and at the remaining two sites no birds were observed during the count. The mean count at confirmed night roosts was 64.3 ± 74.1 birds, the maximum roost count was 202 birds and the total number of birds counted across all sites in Nicaragua was 692 birds. Only four roosts in Nicaragua had 50 or more birds; all of these were located on the island of Ometepe in Lake Nicaragua (Figure 1). There was no difference between Costa Rica and Nicaragua in mean roost size counted in 2016 (t = 0.2, df = 36, P < 0.86). We conducted counts in 2008, 2009, 2011 and 2014 at one of the Nicaraguan roosts included in our 2016 survey, Río Sapoá (Table 1). These counts ranged from 10 to 15 birds; in 2016 we counted 10 birds, which is at the lower end of the historic range of counts.
Estimated reproductive success
We collected data on group size at 27 sites in 2016 to assess the proportion of pairs that successfully fledged young that year. Group sizes ranged from one to five birds. The percentage of groups in Costa Rica containing more than two birds, which possibly represent pairs with one or more recently fledged young, ranged from 0 to 31% across 10 sites, with a mean per site of 15 ± 11.4% of pairs. The proportion of groups containing young in Nicaragua ranged from 0 to 100% across 17 sites with a mean of 45 ± 31% of pairs. The difference between the two countries in mean proportion of groups containing young was statistically significant (t = 3.4, df = 22, P < 0.005). Across both neighbouring countries, however, only 120 groups consisted of more than two individuals while 498 groups consisted of pairs only, suggesting that only 24% of groups consisted of pairs accompanied by recently fledged young. Forty-nine of these 120 putative family groups (41%) were observed at the five roosts located on the island of Ometepe in Nicaragua. There was no relationship between the size of the roost and the percentage of pairs observed with young (linear regression, r 2 = 0.04, df = 24, F = 0.9, P = 0.34).
Roost characteristics
We recorded the elevation, roost substrate and proximity to human habitation of each surveyed roost. The elevation of confirmed roosts in Costa Rica ranged from sea level to 286 m with a mean of 80.8 ± 83.7 m. The elevation of confirmed roosts in Nicaragua ranged from sea level to 682 m, with a mean of 127 ± 192.4 m. The difference in mean roost elevation between the two countries was not significant (t = 1.0, df = 23, P < 0.4), and all but one roost was located below 300 m. Roosts were most likely to be located in mixed-deciduous forest. In Costa Rica, four of 13 (30%) confirmed roosts counted in 2016 were located in mangroves; the remaining 70% were located in mixed deciduous forest. In Nicaragua, only one of 10 (10%) confirmed roosts was located in mangroves and the remaining 90% were in mixed deciduous forest. The proportional use of these two classes of roost substrate did not differ between the two countries (Fisher’s exact test, P = 0.33). In Costa Rica, 11 of the 13 (85%) of the confirmed roosts in 2016 were located within 100 m of human habitation, while in Nicaragua six of 10 (60%) of the confirmed roosts were within 100 m of human habitation. The difference between the two countries was not significant (Fisher’s exact test, P = 0.34).
There was a significant difference in mean roost size between roosts located within 100 m of human habitation versus farther away, with roosts within 100 m of human habitation having larger mean roost sizes (77 ± 74 vs. 30 ± 27 birds; t = 2.2, df = 20.8, P < 0.04). There was no association between roost elevation and roost size (linear regression, r 2 = 0.006, df = 22, F = 0.1, P = 0.72), nor did mean roost size differ between roosts located in mangroves versus mixed-deciduous forest (t = 0.7, df = 9.7, P < 0.52)
Discussion
Our surveys provide a robust assessment of current population sizes of the Yellow-naped Amazon on the Pacific slope of Costa Rica and Nicaragua and an estimate of temporal trends of populations in Costa Rica. Both assessments are cause for substantial concern regarding the health of populations of this endangered species. By every metric we measured, populations in Costa Rica and Nicaragua are small, have declined in the last decade, and are likely to continue to do so given our estimate of reproductive success. Below we summarise these trends, compare them to those seen in other Neotropical parrot species, and discuss likely factors contributing to a rapid population decline of this species in Costa Rica and Nicaragua. We conclude with a list of recommendations for conservation action.
Population trends
In 2016, we observed 990 birds distributed across 21 sites in Costa Rica, and 692 birds distributed across 17 sites in Nicaragua, for a total of only 1,682 birds. The majority of roosts observed in 2016 had fewer than 50 birds, and a direct comparison of 12 roosts counted in 2004 and 2016 showed a 54% decline in mean roost size over the 11-year period. Matuzak and Brightsmith (Reference Matuzak and Brightsmith2007) estimated that during their study the Curú roost consisted of around 300 birds; in our survey 12 years later we counted only 17 birds, representing a 95% decline in numbers at this roost. The rapid decline at many roosts, low overall population numbers and the small average size of most local populations are all cause for significant concern about the long-term viability of this species at the southern portion of its Central American range. There are a few exceptions to these trends. The most notable are the roosts observed on the island of Ometepe in Lake Nicaragua, where the average roost size was 113 birds, three of the five roosts with more than 100 birds in our survey were located, and 41% of all pairs with young were observed. As discussed below though, this location is vulnerable to the same factors that are driving declines elsewhere in Nicaragua and Costa Rica. The other possible exceptions are two mangrove areas deep within the large and well-protected Área de Conservación Guanacaste, which we were not able to access during our survey but are reported by park officials to each contain a large roost of 200–300 birds (T. Lewis pers. obs). These exceptional areas notwithstanding, our results should serve as a clear warning that this species is increasingly vulnerable to extinction in the southern portion of its range, an area typically thought of as its stronghold (BirdLife International 2016b; 2017). It now appears that populations here are following the path towards decline and local extirpation already seen in more northern parts of its range, including El Salvador, Guatemala, Honduras and Mexico (Eisermann Reference Eisermann2003, Marin-Togo et al. Reference Marin-Togo, Monterrubio-Rico, Renton, Rubio-Rocha, Macias-Caballero, Ortega-Rodriguez and Cancino-Murillo2012, BirdLife International 2017).
It is important to note some limitations to our survey approach. First, we counted each roost once, thus our estimates are subject to any day-to day variability in the numbers of individuals attending a given roost. Cougill and Marsden (Reference Cougill and Marsden2004) found considerable day-to-day variability in the size of a single roost of Red-Tailed Amazons, but noted that the greatest variability in size was between seasons, with the peak numbers observed in the post-breeding season when both adults and recently fledged young attend roosts. Our surveys were conducted over 37 days within the immediate post-breeding season, suggesting our estimates are not subject to seasonal variation and should approximate the actual number of birds in the region. Second, we generally surveyed neighbouring roosts on consecutive days, thus some error could be introduced if birds frequently move between these roosts, but radiotracking data from this population suggests that such roost switching is relatively infrequent (Salinas-Melgoza et al. Reference Salinas-Melgoza, Salinas-Melgoza and Wright2013). Third, we may not have counted all roosts within the survey area. We relied on a variety of approaches, including over 20 years of personal experience working with this species in this region, to locate roosts. We are aware of two roosts in the mangrove areas deep within the Área de Conservación Guanacaste in Costa Rica and an island population on the Islas Solentiname in Nicaragua that are difficult to access and thus were not included in the survey, but aside from these we are confident that we have counted most major roosts within the surveyed region.
Our estimates of reproductive success should be interpreted with caution. We found that on average 24% of groups flying to or from roosts consisted of three or more birds, suggesting that, at most, one quarter of the population of adult pairs is successfully reproducing. This proportion is roughly similar to those seen in other studies using similar methodology. A study conducted at the Curú site in Costa Rica in the post-breeding seasons found that 17–18% of groups flying to or from the roost consisted of pairs with young (Matuzak and Brightsmith Reference Matuzak and Brightsmith2007), while counts of Red-tailed Amazons at a single roost over an entire year throughout found 18% of groups flying to roosts and 24% of groups flying from roosts consisted of three or more individuals (Cougill and Marsden Reference Cougill and Marsden2004). It is important to evaluate, however, the critical assumption that groups of three or more birds consist of mated pairs and their recently fledged young. While our observations suggest this is often the case, this estimate is best viewed as a theoretical best estimate of reproductive success. Our own data on nesting success from nest monitoring conducted from 1999 to 2008 in some of the same Costa Rican populations surveyed here found that only 11% of nests successfully fledged young, with most of the mortality arising from nest poaching (Dahlin et al. Reference Dahlin, Chelsea, Rising and Wright2018). Nesting success is higher in populations of the sister species the Yellow-headed Amazon, Amazona oratrix, where 49 of 155 (31%) of nests monitored within or adjacent to protected areas over two nesting seasons from 2016-2017 successfully fledged young, but only limited roost surveys have been conducted in these populations due to an absence of stable roosting sites (C. Britt unpubl. data, F. Tarazona and C. Britt unpubl. data). Direct comparisons between data from nest monitoring and roost surveys are needed to fully evaluate whether the more expedient method of estimating reproductive success from group sizes at roost counts provides an accurate measure of this critical demographic variable.
Factors contributing to decline
Although our study was not designed to directly measure the impact of various factors on Yellow-naped Amazon populations, there is substantial evidence from this and other studies that two factors are primarily responsible for the decline: capture for the pet trade and conversion of habitat for intensive agriculture. The tropical dry forest ecosystem that is the predominant habitat of the Yellow-naped Amazon has long been heavily impacted by anthropogenic disturbance (Janzen and Hallwachs Reference Janzen, Hallwachs and Kappelle2016). For the past 200 years this disturbance was primarily in the form of low-intensity cattle grazing and subsistence farming. Yellow-naped Amazons appear quite tolerant of low-level disturbance; in our surveys most roosts were found in secondary deciduous forest, and many were in close proximity to human habitation; we actually found that mean roost size was larger for roosts located within 100 m of human habitation than for those located farther away. Furthermore, our observations suggest that Yellow-naped Amazons are capable of, and may actually prefer, to nest in more open landscapes such as those maintained by cattle ranching or controlled burns (Dahlin et al. Reference Dahlin, Chelsea, Rising and Wright2018). Thus it is not human activity in general, but rather specific forms of human activity that are driving the observed declines in the Yellow-naped Amazon.
The massive scale of the global pet trade in Neotropical parrots has been recognised for some time (Wright et al. Reference Wright, Toft, Enkerlin-Hoeflich, Gonzalez-Elizondo, Albornoz, Rodríguez-Ferraro, Rojas-Suárez, Sanz, Trujillo, Beissinger, Berovides, Gálvez, Brice, Joyner, Eberhard, Gilardi, Koenig, Stoleson, Martuscelli, Meyers, Renton, Rodríguez, Sosa-Asanza, Vilella and Wiley2001) and is implicated in declines of a great number of species (Cockle et al. Reference Cockle, Capuzzi, Bodrati, Clay, del Castillo, Velázquez, Areta, Farina and Farina2007, Marin-Togo et al. Reference Marin-Togo, Monterrubio-Rico, Renton, Rubio-Rocha, Macias-Caballero, Ortega-Rodriguez and Cancino-Murillo2012, Clarke and de By Reference Clarke and de By2013). In the case of the Yellow-naped Amazon, both their tolerance for close proximity to humans and their well-developed vocal mimicry abilities have rendered them especially vulnerable to the parrot trade. In the past, much of this trade was driven by demand in developed countries (Wright et al. Reference Wright, Toft, Enkerlin-Hoeflich, Gonzalez-Elizondo, Albornoz, Rodríguez-Ferraro, Rojas-Suárez, Sanz, Trujillo, Beissinger, Berovides, Gálvez, Brice, Joyner, Eberhard, Gilardi, Koenig, Stoleson, Martuscelli, Meyers, Renton, Rodríguez, Sosa-Asanza, Vilella and Wiley2001, Vall-Ilosera and Cassey Reference Vall-Ilosera and Cassey2017), but now there is increasing evidence that demand from internal markets within Latin America is driving the poaching of this species and others (Drews Reference Drews, Salem and Rowan2003, Daut et al. Reference Daut, Brightsmith, Mendoza, Puhakka and Peterson2015, Pires Reference Pires2015, Pires et al. Reference Pires, Schneider, Herrera and Tella2016, Berkunsky et al. Reference Berkunsky, Quillfeldt, Brightsmith, Abbud, Aguilar, Aleman-Zelaya, Aramburu, Ariash, McNab, Balsby, Barberena, Beissinger, Rosales, Berg, Bianchi, Blanco, Bodrati, Bonilla-Ruz, Botero-Delgadillo, Canavelli, Caparroz, Cepeda, Chassot, Cinta-Magallon, Cockle, Daniele, de Araujo, de Barbosa, de Moura, Del Castillo, Diaz, Diaz-Luque, Douglas, Rodriguez, Garcia-Anleu, Gilardi, Grilli, Guix, Hernandez, Hernandez-Munoz, Hiraldo, Horstman, Portillo, Isacch, Jimenez, Joynerak, Juarez, Kacoliris, Kanaan, Klemann, Latta, Lee, Lesterhuis, Lezama-Lopez, Lugarini, Marateo, Marinelli, Martinez, McReynolds, Urbinaat, Monge-Arias, Monterrubio-Rico, Nunes, Nunes, Olaciregui, Ortega-Arguelles, Pacifico, Pagano, Politi, Ponce-Santizo, Reyes, Prestes, Presti, Renton, Reyes-Macedo, Ringler, Rivera, Rodriguez-Ferraro, Rojas-Valverde, Rojas-Llanos, Rubio-Rocha, Saidenberg, Salinas-Melgoza, Sanz, Schaefer, Scherer-Neto, Seixas, Serafini, Silveira, Sipinski, Somenzari, Susanibar, Tella, Torres-Sovero, Trofino-Falasco, Vargas-Rodriguez, Vazquez-Reyes, White, Williams, Zarza and Masello2017). Our anecdotal but frequent observation of pet Yellow-naped Amazons in both Costa Rica and Nicaragua suggest this trade is an ongoing threat in these countries (C. Dahlin, M. Lezama López, and T. Wright pers. obs.).
Habitat loss is commonly reported as contributing to the decline of many Neotropical parrots (Snyder et al. Reference Snyder, McGowan, Gilardi and Grajal2000, Clarke and de By Reference Clarke and de By2013, BirdLife International 2016a). In the case of the Yellow-naped Amazon, recent changes in land-use and conservation practices in both Costa Rica and Nicaragua are likely contributors to population declines. Over the last 40 years, Costa Rica has placed large portions of its territory into regional Áreas de Conservación that are managed for biodiversity protection and ecosystem services. Over the last 25 years we have commonly seen birds moving between these protected areas and the low-intensity agro-landscape surrounding it (T. Wright and C. Dahlin, pers. obs.) and another study documented extensive feeding by Yellow-naped Amazons in human-modified areas at the Curú site in Costa Rica (Matuzak et al. Reference Matuzak, Bezy and Brightsmith2008). In the last decade, however, these agro-landscapes have been increasingly converted from ranching to higher-intensity uses such as rice and sugar cane farming; Yellow-naped Amazons are now rarely seen in these high-intensity agricultural areas which lack suitable trees for nesting and feeding (C. Dahlin and T. Wright pers. obs.). Nicaragua has also suffered widespread loss of tropical dry forest habitat to high-intensity agriculture, and of mangrove habitat to shrimp farming and salt production (Tarrason et al. Reference Tarrason, Urrutia, Ravera, Herrera, Andres and Espelta2010, Aide et al. Reference Aide, Clark, Grau, López-Carr, Levy, Redo, Bonilla-Moheno, Riner, Andrade-Nuñez and Muñiz2013, Benessaiah and Sengupta Reference Benessaiah and Sengupta2014). As a result, on the Pacific slope of Nicaragua, Yellow-naped Amazons are virtually absent from the lowlands and are now found mostly on the upper slopes of volcanoes where some forest has been preserved as national parks and reserves, and on islands within Lake Nicaragua where there is less intensive agriculture. One bright spot in Nicaragua is the recent strengthening of a system of private reserves that are incentivised by the national government to protect and restore native habitat. If this trend can be maintained it could eventually result in an increase in the availability of suitable habitat for this species on the Pacific slope of Nicaragua (D. Hille, D. Wiedenfeld, M. Lezama López, D. Brightsmith & M. Patten unpubl. data).
Other factors may also be contributing to the decline of the Yellow-naped Amazon. Global climate change has resulted in gradual increases in temperature and declines in rainfall in the Pacific slope for Costa Rica and Nicaragua (Hidalgo et al. Reference Hidalgo, Amador, Alfaro and Quesada2013); these changes may affect the timing and amount of the fruits and seeds available for Yellow-naped Amazons. Perhaps more immediate, though, are Allee effects, which can cause a population’s growth rate to become negative when population size falls beneath a certain threshold (Courchamp et al. Reference Courchamp, Berec and Gascoigne2008). In social species, these can occur when groups are too small to offer protection from predators, when suitable mates become scarce, or when opportunities for cooperative interactions such as sharing public information about food are rare (Courchamp et al. Reference Courchamp, Berec and Gascoigne2008). Rapid declines have been reported in a wide range of parrot species, including such iconic species as the Kakapo Strigops habroptilus (Bergner et al. Reference Bergner, Dussex, Jamieson and Robertson2016), African Grey Parrot Psittacus erithacus (Annorbah et al. Reference Annorbah, Collar and Marsden2016), Swift Parrot Lathamus discolor (Heinsohn et al. Reference Heinsohn, Webb, Lacy, Terauds, Alderman and Stojanovic2015), and the Carolina Parakeet Conuropsis carolensis (Snyder Reference Snyder2004). While in most cases factors such as capture for the pet trade, habitat loss, or introduced predators are implicated in the initial population decline, Allee effects are suspected to have accelerated the pace of population collapse in many of these species (Snyder Reference Snyder2004). Certainly, the possibility of Allee effects should be considered very seriously in a social species like the Yellow-naped Amazon. While we did not find a relationship between roost size and the proportion of groups that likely represented pairs and recently fledged young, we only found 120 putative family groups in our survey and 49 of these were from the roosts on Ometepe. Rapid declines and population crashes have already been reported for this species in several parts of its range (BirdLife International 2017). In this context, the recent population decline we document at the Curú site and the fact that over 50% of the roosts we surveyed in Costa Rica and Nicaragua had 20 or fewer birds is further cause for serious alarm. Our data will provide a critical baseline for future work addressing the important question of whether small populations of this species are especially vulnerable to rapid declines due to Allee effects or global climate change.
Conservation action recommendations
In December 2017, IUCN upgraded the status of this species from ‘Vulnerable’ to ‘Endangered’. The data presented here, which show a > 50% decline in population at many roosts over the last 11 years, strongly support this change in conservation status and emphasise the need for rapid action before this species experiences further declines. The possession of wild animals, including parrots, is now illegal and subject to hefty fines in Costa Rica (Anonymous 2013b). Continued efforts at enforcing these regulations, coupled with substantive education programs to raise public awareness of the importance of parrot conservation, are recommended to reduce endemic poaching in Costa Rica. Similar regulations and education efforts are recommended for Nicaragua, where Yellow-naped Amazon populations are generally smaller, with the notable exception of Ometepe, which still hosts sizeable populations that merit special protection. Captive breeding and reintroduction programmes could also be valuable, particularly if they target areas with adequate habitat and protection of nests. In the longer term, conservation and restoration of dry forest habitat with preservation of large native trees required for nesting cavities will be important, particularly in Nicaragua, which has had more extensive deforestation and more limited restoration efforts. Further development of collaborations between conservationists, landowners and reserve managers will be critical for preserving this iconic parrot species in the southern portion of its range. On a larger scale, there is a dearth of reliable data on current populations of this species in the remainder of its range (BirdLife International 2016b). Standardised surveys conducted with similar methodology in the remainder of the Yellow-naped Amazon’s range in north-eastern Nicaragua, El Salvador, Honduras, Guatemala and southern Mexico would provide an improved picture of the overall status of this species and help target both studies of the factors impacting populations, and conservation strategies to improve their health.
Acknowledgements
We thank Sophie Nazeri, Molly Dupin, Alesa Trimeloni, Dominique Hellmich, Norland Zambrano, José Martín Vallecillo, and Orlando Jarquín for assistance with 2016 roost counts, Hugo Guadamuz Rojas and Sarah Garcia for assistance with 2005 roost counts, Hugo Guadamuz Rojas for assistance with the original roost survey in 1994, and Sophie Nazeri for extracting data on 2005 and 1994 roost counts from field notebooks. We gratefully acknowledge Roger Blanco of the Área de Conservacíon Guanacaste in Costa Rica, Roberto Zuñiga of the Área de Conservacíon Tempisque in Costa Rica, Myrna Moncada of the Red de Reservas Silvestres Privadas in Nicaragua, Carlos R. Mejía of the Ministerio del Ambiente y Recursos Naturales in Nicaragua and their staff for facilitating this research, and the many landowners in in Costa Rica and Nicaragua for their support of our work and dedication to the protection of biodiversity. Funding was provided by the University of Pittsburgh Central Research Development Fund (C. R. D., grant number 9009626), New Mexico State University Manassee Scholar Award (T. F. W.), the Ara Project (T. L.) and the World Parrot Trust (T. L.).