According to the World Health Organization in 2020, around 50 million people have dementia worldwide. This number is projected to reach 82 million in 2030 and 152 million in 2050. Mild cognitive impairment (MCI) is considered to be an intermediate, prodromal phase of dementia. Its prevalence will increase with the predicted ‘dementia avalanche’ due to increasing life expectancy.Reference Petersen, Lopez, Armstrong, Getchius, Ganguli and Gloss1
MCI is a heterogenous syndrome characterised by deficits in cognitive functioning (i.e. learning and memory, language, executive functioning, attention and social cognition) without significant impairment in instrumental activities of daily living.Reference Dunne, Aarsland, O'Brien, Ballard, Banerjee and Fox2 Although persons with MCI (PwMCI) are able to function independently, they often require some assistance with complex activities. This can result in social, emotional and functional challenges and consequent caregiver burden.Reference Dean and Wilcock3 The issue of caregiver burden has been extensively explored in dementia; however, caregiver burden may also be a significant issue in MCI, and the PwMCI–carer dyad should be supported for better outcomes.Reference Domingues, Verreault and Hudon4–Reference Connors, Seeher, Teixeira-Pinto, Woodward, Ames and Brodaty6 The recent Manchester consensus group for MCI recommends exploring the psychosocial impact of receiving a diagnosis of MCI on patients and carers.Reference Dunne, Aarsland, O'Brien, Ballard, Banerjee and Fox2
A substantial proportion of individuals seen in memory clinics are diagnosed with MCI; however, we have little objective data on the psychosocial impact of the diagnosis for these patients. In order to address this knowledge gap we designed a cross-sectional, cohort study. The study had the following aims.
(a) To examine the perspectives of PwMCI and family members and/or friends on their experience of receiving the results of an assessment at the memory clinic, including their recall and understanding of the diagnosis.
(b) To explore emotional well-being and changes in attitude to health and lifestyle following the diagnosis.
(c) To explore and understand the needs of the PwMCI and their family members.
Method
Service description
St James's Hospital (SJH) Memory Clinic provides multidisciplinary diagnostic assessments of complex cognitive presentations. The service receives referrals from multiple sources, both local and national, for primary and secondary opinions. Assessment at SJH Memory Clinic entails a comprehensive interview, collateral history and physical examination by a medical doctor, carried out in conjunction with neuropsychological testing. Diagnostic investigations including blood testing and neuroimaging (typically magnetic resonance imaging) are completed for all patients. Cases are discussed at a multidisciplinary consensus meeting, where expert opinions from neurology, geriatrics, psychiatry and neuropsychology are discussed to arrive at a diagnosis. Where diagnosis remains unclear or debatable, more in-depth investigation (such as cerebrospinal fluid biomarkers, functional neuroimaging or further targeted neuropsychological testing) is completed.
SJH Memory Clinic uses the MCI diagnostic label cautiously. The clinicians strive to further subtype MCI as amnestic or non-amnestic, single domain or multiple domain, with a comment on risk of progression.
The results of the assessment and associated recommendations are discussed with the patient at a face-to-face or telephone feedback meeting, depending on the patient's preferences and the complexity of the case. Patients are advised, if possible, to bring a family member to the meeting, and personalised written results and recommendations are also provided at the meeting for future reference.
At the feedback meeting, patients are advised about addressing potentially modifiable risk factors that can prevent or slow cognitive decline. The discussion focusses on well-described brain health measures. This includes evaluating lifestyle profile (activity, diet, smoking, alcohol use), social circumstances, vascular risk factors (hypertension, diabetes), sensory function (hearing, vision) and mental health (depression). Social prescribing networks and wellness programmes are signposted. Patients are also given written information about brain health measures.
Study methodology
Ethical approval for the study was granted by SJH and Tallaght University Hospital Research Ethics Committee (project ID: 0344). The study was completed with patients who had attended the Memory Clinic for an assessment from 1 January 2020 to 30 April 2021 and had received a diagnosis of mild cognitive impairment, together with their nominated family member or friend. A time period of more than 12 months was used to account for the times when the Memory Clinic was not fully operational owing to COVID-19 restrictions. A list of the names of patients who had received a diagnosis of MCI (the PwMCI) was drawn from the clinic database. A patient information leaflet explaining the purpose and details of the study, along with a consent form, was posted to patients who had given consent to be contacted in the future for participation in research by the Memory Clinic team. A week later, the PwMCI were contacted by a member of the team to answer any questions about the study, obtain verbal consent and set a suitable date and time for a 15-min phone call to complete the questionnaire. Also, with patients’ consent, details of the family member or friend who was most involved in their lives and most familiar with their clinic evaluation were obtained, so that this individual could be invited to complete the carer questionnaire. The patients were requested to complete and sign the consent form and post it to the memory clinic using the stamped and addressed envelope sent to them. For the purpose of consistency, family members and friends who support PwMCI are referred to here as ‘carers’, although they may not have regarded themselves as such. We also use ‘FwMCI’ to refer to family members or friends of PwMCI. Information leaflets explaining the study and inviting FwMCI to participate were posted to the given addresses along with the consent forms. The same steps as described above were followed for FwMCI to complete the questionnaire over the phone. If a patient did not give consent to contact a family member or friend, or the family member or friend declined to participate in the study, then only the PwMCI's responses were included in the data-set for that case.
A questionnaire was formulated (using Microsoft Word, version 16, Windows) by iterative design, and the following variables were explored and recorded:
(a) Demographic details including age, gender, marital status, level of education and employment.
(b) Recall and understanding of the diagnosis and advice given following assessment at the memory clinic.
(c) Changes in life following the diagnosis, including lifestyle changes following doctor's recommendations.
(d) Psychological impact of the diagnosis, determined by enquiring about mood, self-esteem, and continued participation in enjoyable activities and by completing the Geriatric Anxiety Inventory – Short Form (sensitivity 75%, specificity 87% for anxiety disorders).Reference Byrne and Pachana7
(e) Identification of unmet needs e.g. more information about the diagnosis, support to implement advised lifestyle changes and future planning.
A similar questionnaire was formulated for FwMCI to record demographic details, recall and understanding of the diagnosis, degree of involvement with PwMCI and Zarit Burden Scale to assess carer burden.Reference Zarit, Reever and Bach-Peterson8 FwMCI were requested to complete and return the Zarit Burden Interview along with the consent form, and the rest of the questionnaire was completed over the phone.
This study used mixed methods, involving quantitative and qualitative data. The interviewer selected the most accurate option based on the person's response for each of the questions; if none of the options applied then the person's response was written down verbatim next to the ‘other’ option. Data were initially collected on study datasheets during the phone call, coded and then transferred to a Microsoft Excel (version 16, Windows) spreadsheet for analysis.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
A total of 250 new patient assessments were carried out in the Memory Clinic from 1 January 2020 to 30 April 2021. Ninety-five patients (38%) received a diagnosis of MCI. Figure 1 details the recruitment of the participants for the study. Forty-seven PwMCI participated in the study (response rate: 68.1%).

Fig. 1 Flowchart detailing participant recruitment. MCI, mild cognitive impairment; PwMCI, person with MCI; FwMCI, friend or family member of person with MCI.
Patient characteristics
The demographic characteristics of the PwMCI cohort are presented in Table 1. The mean age of the participants was 71.6 years, and the median age was 71.0 years (range: 54 to 92 years). Most of the participants were retired (66%, n = 31). Only 8.5% of PwMCI (n = 4) reported the diagnosis affecting their working status. Of these, two took early retirement, one changed post and one reduced their working hours.
Table 1 Demographics of patients with mild cognitive impairment

Most participants (59.6%, n = 28) had no personal experience of dementia through a diagnosis of a family member or friend. In most cases, the referral to the Memory Clinic was prompted by another person, i.e. a concerned family member (34%, n = 16), concerned general practitioner (GP) (25.5%, n = 12), another specialist (12.8%, n = 6) or a friend (n = 1), or following an in-patient stay (n = 1). In about one-fifth of the cases (n = 10) was the referral initiated owing to the PwMCI's own concerns. No correlation was found between observation of a relative or friend with memory problems and seeking a self-referral (r = 0.13).
On enquiry, most of the PwMCI were satisfied with their current health state and perceived it to be in the good-to-excellent range (excellent n = 5, very good n = 12, good n = 14), followed by 27.7% (n = 13) of PwMCI who reported it as fair. Only 6.4% (n = 3) perceived their current health to be poor. Most PwMCI (66%, n = 31) did not report a history of mental illness, and others were currently receiving treatment from a psychiatrist or community mental health team (19.2%, n = 9) or their GP (14.9%, n = 7).
Experience of the memory clinic feedback appointment
On enquiry about the outcomes of the assessment, most participants (59.6%, n = 28) did not recall the diagnosis of MCI or a medical construct to explain their presentation. Many PwMCI (34.0%, n = 16) did not remember the details of the results discussed and did not view their problems as arising from a disease process. Only 21.3% (n = 10) of PwMCI used the term ‘mild cognitive impairment’ to describe their cognitive difficulties; 14.9% (n = 7) described these as ‘mild memory problems’, one participant said ‘mild impairment’ and one said ‘early dementia’. Most participants (57.4%) remembered discussing their results at a face-to-face meeting either with the doctor (n = 20) or a doctor and social worker (n = 7). Others said that the results were discussed by a doctor over the phone (n = 7, 14.9%), two participants said that they only received written summary of their assessment in the post and eight participants did not remember the format of feedback. There was no correlation found between face-to-face meeting and accurate recall of the diagnosis as ‘mild cognitive impairment’ (r = 0.3).
Only a quarter of the PwMCI (n = 12) viewed their problems as pathological and attributed them to a likely cause or narrative. The causes described included ‘brain haemorrhages’, ‘ECT related’, ‘amyloid in the brain’, ‘few different things’, ‘anxiety’, and ‘tablets prescribed for depression’. Many considered it to be due to ageing. More than half of PwMCI (60%, n = 28) said that likely causes were not discussed, and three participants said that they ‘don't remember’.
The participants had a varied understanding of the likelihood of progression of their difficulties. Most said that the chances of progression were not discussed with them (32%, n = 15) or that they did not remember whether it was discussed (29.8%, n = 14). About one-fifth of the PwMCI believed that their problems were unlikely to progress, and only four respondents understood that progression is determined by many factors. A similar number (n = 4) believed that their difficulties were highly likely to progress to dementia.
‘Relief that it's not dementia’ was the most common reaction of PwMCI to the results (34.0%, n = 16). Others said that they were glad to know the likely cause of their difficulties (14.9%, n = 7), six participants (12.8%) were shocked that their problems were more serious than they had anticipated and one participant reported being stigmatised with a label.
Most PwMCI (n = 30, 63.8%) were satisfied with the process of disclosure and with the advice given to manage their problems. The majority of the participants discussed the results with family only (53.2%, n = 25), followed by both family and friends (36.2%, n = 17). Some participants did not share the results of their assessment with friends or family (8.5%, n = 4). One participant said that this was owing to feeling embarrassed, and others said that there was ‘no diagnosis to share’.
When asked for suggestions for improving the diagnostic and disclosure process, only 17.0% (n = 8) of PwMCI had something to say. Some suggestions were as follows.
‘Be definite and not just say it might not be that’
‘Would have preferred to receive the written personalised feedback form before the face-to-face meeting. It would have given me time to process the information and prepare questions. Final report that is sent to the GP should also be sent to the patient’
‘The team should keep in contact more frequently rather than just a yearly review’
Lifestyle changes after the diagnosis
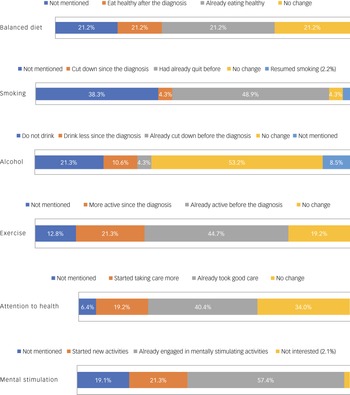
Figure 2 summarises the responses of the participants when asked about various aspects of their lifestyle following their attendance at the Memory Clinic and receiving brain health advice. ‘Doing crosswords’ and ‘reading’ were the most common cited mentally stimulating activities by PwMCI. When asked about new activities since the diagnosis, PwMCI had taken up golf (n = 1), playing football with kids (n = 1), writing (n = 1), doing arts (n = 1), crosswords and games (n = 1), reading and mindfulness (n = 2), resuming language classes (n = 1) and socialising with friends (n = 1).

Fig. 2 Lifestyle changes after diagnosis.
Most of the participants (57.4%, n = 27) said that they had continued to drive with no problems since the diagnosis, three said that they are more cautious now and only one had quit driving since the diagnosis.
Awareness of hearing and vision as potentially modifiable risk factors was also explored. Most (55.3%) either didn't remember (n = 12) having a hearing screen during the assessment visit or said that it was not undertaken (n = 14). Among the others, 20 (42.6%) participants recalled having a hearing screen, of which six said that they were advised to have further audiology assessment. Only two PwMCI said that they were prescribed hearing aids following the audiology assessment, and only one participant reported wearing them all the time. Very few participants (17.0%, n = 8) recalled being informed that uncorrected visual problems can adversely affect the memory, and only one of them scheduled an optician review to mitigate the risk.
Future planning
Many participants (61.7%, n = 29) had already written their Wills before the diagnosis. Only 10.6% (n = 5) proceeded to write their Will after the diagnosis. No correlation was seen between PwMCI perceiving that their condition can worsen in the future and writing a Will (r = 0.12). The attitude towards enduring power of attorney (EPOA) was varied. Many did not understand its significance (n = 16, 34.0%), others said that they had not set it up yet (n = 12, 25.5%) and only five PwMCI set up an EPOA after their diagnosis. Most participants (n = 44, 93.6%) did not make any changes to their living arrangements, and two participants said that they had been spending more time with their family since the diagnosis.
Psychological impact
Most PwMCI (n = 28, 59.6%) responded by saying that the diagnosis had not affected their mood or self-esteem, and eleven participants (23.4%) acknowledged an effect to some extent. Most participants shared the views that the diagnosis probably had not changed the way other people see them (n = 31, 70%), that it was not affecting people close to them (n = 33, 70.2%), that they could control the progress of their problems (n = 34, 72.3%) and that they had not stopped doing anything they enjoyed since the diagnosis (n = 42, 89.4%). About one-fifth of PwMCI (n = 10) scored ≥3 on the Geriatric Anxiety Inventory – Short Form (sensitivity 75%, specificity 87% for anxiety disorders).Reference Byrne and Pachana7
Family members study
The family member/friend part of the study could only be completed in 36 cases (Fig. 1). The mean age of FwMCI was 60.6 years and the median was 61.5 years. Seventy-eight per cent of the respondents (n = 28) were female, and the relationship of the respondents with PwMCI included spouse or partner (n = 18), child (n = 11), sibling (n = 6) and neighbour (n = 1). Twenty FwMCI (55.6%) were living with the PwMCI. Fourteen were working full time and five part time, and twelve were retired. Four FwMCI reported a change in their employment status since the diagnosis, and it was related to the diagnosis in three cases. Fifty-eight per cent of the respondents (n = 21) said that they also had other relatives or friends with memory problems or dementia.
Experience of the memory clinic feedback visit
Thirteen respondents (36.1%) attended a face-to-face feedback meeting at the Memory Clinic with PwMCI; seven (19.4%) were informed of the outcome of the assessment by the PwMCI themselves, and 12 (33.3%) were informed by the doctor over the phone, of whom four also read the written summary of the results sent out to the PwMCI in post. About half the FwMCI recalled the diagnosis as ‘mild cognitive impairment’ (n = 15); others recalled it as ‘minor cognitive impairment’ (n = 1), ‘cognitive impairment’ (n = 1) or ‘cognitive dysfunction’.Reference Petersen, Lopez, Armstrong, Getchius, Ganguli and Gloss1 About a quarter (n = 9) of the FwMCI said that they did not really know the diagnosis. Most participants had no insight into the likely cause(s) of PwMCI presentation: ‘wasn't told’ (n = 14), ‘the doctors do not know yet’ (n = 7), ‘cannot recall’ (n = 1). Fifteen (41.6%) FwMCI said that they were not informed about the chances of progression, and only a quarter of the respondents displayed an understanding of the possibility of the cognitive issues progressing.
About a third (n = 13) of FwMCI said that they were relieved that it was not dementia, whereas others expressed feeling reassured, surprised or shocked on hearing the results. Most FwMCI (n = 22, 61.1%) were satisfied with the information and advice given at the Memory Clinic. There were some suggestions for improvement that included providing more explanation and support to the family, clarity around role of medications and follow-up and signposting local supports and services suitable for the PwMCI at this stage.
Change in responsibilities
Sixteen FwMCI took up some responsibilities of the PwMCI around the time of the diagnosis. This most commonly involved financial planning (n = 8); other responsibilities included cooking, driving, medication management and looking after appointments. About the same number mentioned that they started helping the PwMCI more than 2 years ago. Only three PwMCI were receiving any outside help, e.g. carer or family visits. About two-thirds of FwMCI said that they had not discussed future planning or wishes with PwMCI yet. One quarter (n = 9) of FwMCI had additional care responsibilities in the form of caring for other family members or children. Most participants (n = 32) identified potential support, if needed in the future, involving other children, siblings, neighbours or extended family members of PwMCI.
Zarit Burden Interview
Thirty-four FwMCI completed the Zarit Burden Scale. Of those who scored in the burden range, 11 FwMCI reported mild to moderate burden, four reported moderate to severe burden and one reported severe burden.
Discussion
People develop their own narratives to make sense of health states; these are known as ‘illness representations’. Illness representations determine the emotional response to a condition, as well as attitudes and behaviour regarding medical advice and treatment. Understanding these illness representations can guide clinicians about ways to best support their patients and provide personalised care.Reference Clare, Gamble, Martyr, Quinn, Litherland and Morris9,Reference Dempster, Howell and McCorry10
The Common Sense Model (CSM) developed by Leventhal et alReference Leventhal, Phillips and Burns11 proposes five dimensions that influence illness representations: (a) identity, (b) causes, (c) timeline, (d) consequences and (e) control. In our cohort of PwMCI, most of the participants did not recall the diagnosis of MCI for their condition. In addition, very few considered possible causes and fewer still considered that their condition might worsen in the future. Most participants described being in control of the progress of their condition and were relieved that it was ‘not dementia’. Overall, most patients did not report significant psychosocial distress or worry in relation to their MCI diagnosis. It may be argued that MCI is a more abstract illness concept than a diagnosis of dementia; this, in combination with the inherent memory difficulties in PwMCI, may account for the lack of strong MCI illness representation seen in our cohort.
There was slight discordance of illness perception among the PwMCI–FwMCI dyads. About half of FwMCI recalled a diagnosis to explain their loved one's symptoms, and about a quarter of FwMCI displayed reasonable insight into the possibility that the condition could worsen over time. Most FwMCI had limited understanding of the chances of progression or cause(s) of their loved one's condition and were relieved that it was not dementia. The CSM proposes that development of beliefs about a condition or illness is a dynamic process that is influenced by a person's previous experience of illness and treatment, observation of others close to them, social comparison and information from the media. More specifically, evidence suggests that prior personal experience of dementia in family or friends and perceived health status of an individual influences the way they interpret and cope with the MCI diagnosis.Reference Blatchford and Cook12 In our cohort, in contrast to FwMCI, the majority of PwMCI had no previous experience of a person with memory problems. This may explain the discordance in the perception of MCI between the two groups.
We found that a significant minority (21%) of patients diagnosed with MCI had self-instigated referral for assessment: the majority attended based on concern raised by family, friends or healthcare professionals. This is in keeping with our finding that overall FwMCI displayed greater understanding of the diagnosis of MCI than PwMCI themselves.
Beliefs about illness causality affect health-related behaviours and emotional response to an illness.Reference Rodakowski, Schulz, Gentry, Garand and Lingler13 Most participants in both groups did not attribute a likely cause to the PwMCI's presentation, and many considered it to be due to ageing. This is consistent with findings of earlier studies.Reference Lin, Gleason and Heidrich14 Participants in our study did not attribute MCI to modifiable lifestyle risk factors such as physical inactivity, smoking, obesity, social isolation or poor diet and had limited understanding of the potentially modifiable risk factors for dementia. This is notable, as detailed information leaflets describing MCI and discussing lifestyle interventions are provided at diagnostic feedback meetings. It is possible that the PwMCI may be experiencing ‘information overload’ at these meetings. It is also possible that the PwMCI do not recognise the interventions suggested in the advisory leaflet as relevant to them. Finally, the level of understanding of the diagnosis may be related to the type of cognitive deficits the PwMCI is experiencing. We need to consider more impactful ways of educating PwMCI about modifiable risks. Simply providing information leaflets clearly does not meet their needs.
Very few participants in both groups were concerned about the condition progressing to dementia. This may explain the limited uptake of future planning advice provided at the feedback discussion and detailed in the feedback information pack. According to the CSM framework, a perception that illness is controllable is associated with positive psychological well-being.Reference Hagger and Orbell15 Most PwMCI in our study reported being in control of their memory problems. The majority reported positive psychological well-being and did not endorse symptoms of depression or anxiety. Patients who view their cognitive decline as part of ageing and consider it to be non-pathological score higher on measures of well-being and satisfaction with life.Reference Clare, Gamble, Martyr, Quinn, Litherland and Morris9 This was true for our PwMCI cohort, as many had not pathologised their cognitive problems. This raises the question of whether a greater understanding of the diagnosis of MCI and possibility of progression to dementia may cause more iatrogenic psychological harm than benefit.
Existing evidence suggests that patients’ and carers’ understanding of MCI is influenced by clinicians’ understanding and communication of the construct and quality of information provided.Reference Blatchford and Cook12 Clinicians have varying perspectives about the utility of the ‘mild cognitive impairment’ diagnosis. This influences their discussion of the results of the assessment and the advice given.Reference Saunders, Ritchie, Russ, Muniz-Terrera and Milne16,Reference Visser, van Maurik, Bouwman, Staekenborg, Vreeswijk and Hempenius17 Clinicians from different specialties and with different levels of clinical experience review patients in the Memory Clinic at SJH. The format of feedback meetings also varies depending on both the clinician's judgement and the patient's preferences. For this study, no data about the clinician's specialty and years of experience were collected. Hence, it is not possible to comment on any correlation between the clinician communicating the results and the participant's perspective in this study.
Although this study provides useful insights into the subjective experiences of PwMCI–FwMCI dyads, the results should be interpreted in the light of the study's limitations. The study recruited participants from only one clinic sample, the MCI diagnosis was not further categorised to group participants (type, neuropsychological scores) and the duration of MCI diagnosis varied among the participants. Cognitive changes inherent to MCI can affect a person's understanding and interpretation of the information provided, and participants may forget the information they have been told over time.Reference Yates, Stanyon, Samra and Clare18 Moreover, the study did not account for variation in the communication practices of the clinicians. Although the median age of our study participants was 71 years, this should not have a bearing on the generalisability of the findings, as participants’ ages varied widely. It can be argued that studying an older cohort of PwMCI may result in further insights, owing to complex interactions between cognitive impairment and frailty in older people.
The results of this study indicate several possible improvements for future policy and practice. Clinicians need to be more specific about underlying causes, prognosis and risk reduction interventions when communicating a diagnosis of MCI. It should be clearly stated that the patients’ cognitive difficulties are not normal for their age and education level. Many participants in our study expressed frustration at the lack of available treatments and services suitable for PwMCI. All patients receiving the diagnosis of MCI should be offered post-diagnostic support personalised to their needs in the form of psychosocial interventions and caregiver support.
About one-third of FwMCI in our study reported mild to moderate burden caused by supporting their loved one with MCI. Public education campaigns raising awareness about MCI and modifiable risk factors for dementia can help influence ‘illness representation’ for MCI. Based on our findings, awareness campaigns targeted at family and friends identifying MCI in their loved ones may be more effective than those encouraging people to seek assessment for subjective memory concerns. Timely intervention at the MCI stage can delay the onset of dementia and even improve cognitive abilities, resulting in better quality of life for PwMCI. This will reduce the burden on carers and the healthcare system in caring for people with dementia and potentially result in significant cost savings.
Data availability
The data that support the findings of this study are available from the corresponding author, N.M., upon reasonable request.
Author contributions
N.M. devised the research question, produced the study materials including participant information and consent sheets, collected an equal share of the data, analysed the data, and drafted the manuscript including tables and figures. L.K. collected an equal share of the data and had input into manuscript editing and formatting. M.U. collected an equal share of the data and drafted part of the manuscript, including producing figures. D.B. participated in producing the study materials and collected an equal share of the data. I.B. participated in identifying the eligible participants and producing study materials. D.R. provided oversight of data collection and production of study materials. E.G. provided oversight of study conception, development of study materials, data collection and manuscript production
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.








eLetters
No eLetters have been published for this article.