Introduction
Sjögren's syndrome is a multi-organ connective tissue disorder characterised by xerostomia and xeropthalmia. These symptoms are collectively known as sicca syndrome.Reference Ramos-Casals, Brito-Zerón, Sisó-Almirall and Bosche1 Pathogenesis is poorly understood. However, lymphocytic infiltration propagating multi-system chronic inflammation, particularly of the exocrine glands, appears to be a defining feature. In addition to the glandular manifestations, a broad range of other systems may be implicated, collectively known as extra-glandular features. Examples include vasculitis, peripheral neuropathy, renal tubular acidosis, pulmonary involvement, lymphoproliferative disease and/or immunological abnormalities.Reference Chisholm and Mason2 Like most rheumatological diseases, there is a strong female preponderance. Although geographic variation exists, with European and Asian populations most affected, a 2014 meta-analysis found an overall incidence of 7 per 100 000 globally.Reference Qin, Wang, Yang, Yang, Ma and Huang3
Sjögren's syndrome can occur in a primary form, described as an isolated sicca syndrome, and in a secondary form, where concurrent rheumatological disease exists, most commonly rheumatoid arthritis or systemic lupus erythematosus.Reference Kollert and Fisher4 Diagnosis can be challenging, and encompasses the objective findings of ocular and/or oral dryness and evidence of an underlying autoimmune pathology. Many differential diagnoses exist for sicca syndrome and there is no single diagnostic to determine Sjögren's syndrome, hence classification systems and disease criteria are vital.
In 2016, the American College of Rheumatology and the European League Against Rheumatism jointly endorsed a new set of criteria developed by the Sjögren's International Collaborative Clinical Alliance investigators for the classification of primary Sjögren's syndrome.Reference Mariette and Criswell5,Reference Shiboski, Shiboski, Seror, Criswell, Labetoulle and Lietman6 These guidelines are based on a weighted scoring system, where a score of 4 or more is required to make the diagnosis. In addition, an individual can only be classified as having primary Sjögren's syndrome if they have either of the following two items (each with a score value of 3 points): relevant autoantibodies or a sublabial gland biopsy showing focal lymphocytic sialadenitis.
Despite being considered the foremost investigation for the establishment of primary Sjögren's syndrome, a sublabial gland biopsy is an invasive procedure and is often reserved for those cases where a diagnostic dilemma exists such that appropriate treatment cannot be instigated. A variety of surgical techniques have been described in the literature with varying harvest rates. Typically, four salivary gland lobules are required to definitively diagnose primary Sjögren's syndrome.Reference Daniels and Fox7 Challenges include identifying these glands when using a minimally invasive technique in a highly vascular mucosal surface in a conscious patient under local anaesthetic. Reported complications include bleeding, infection, wound dehiscence, scarring and lower lip numbness. Damage to surrounding nerves with resultant paraesthesia has been noted in up to 11 per cent of cases, often through inadequate recognition of the proximity of the mental nerve to the harvest site.Reference Varela Centelles, Sánchez-Sánchez, Costa-Bouzas, Seoane-Romero, Seoane and Takkouche8
In an effort to make this procedure as seamless, bloodless and safe as possible, our institution adopted the chalazion clamp technique to enhance the identification of pertinent structures in a timely manner. Our initial experience with this variation to the traditional technique was overwhelmingly positive, whereby glands were obtained in all cases and no complications were recorded (n = 23).Reference Wijaya, Ramli and Khoo9 The present study aimed to evaluate the accuracy of this procedure in terms of providing a minimum number of glands required to aid histopathological diagnosis.
Materials and methods
Review process
A retrospective analysis was undertaken in a single tertiary referral centre at an academic institution. All patients who had undergone sublabial gland biopsy between July 2014 and September 2019 were analysed. Inclusion criteria were all patients referred from medical departments requesting gland biopsies for the diagnosis of Sjögren's syndrome, and limited to those who had undergone sublabial gland biopsy with a chalazion clamp, and who had attended a post-operative review clinic. Excluded were patients who underwent biopsies of minor salivary glands harvested in conjunction with the excision of other lip lesions in tandem. Patients who failed to attend the post-operative review clinic, those with missing medical records on post-operative review or patients who were lost to follow up were excluded. Medical records, operative notes and histopathological results were reviewed and data collected.
Operative technique
A standard 9 cm chalazion clamp with a 30 × 20 mm ellipsoid fenestra was utilised on all patients (Figure 1a and b). Gentle traction is applied at the edge of the lower lip using dry gauze, to reflect the lower lip forward and downward for access to the inner lip surface, exposing the mucosal surface where the minor salivary glands are located. The clamp is placed over the reflected lower lip in such a way that the fenestrated leaf of the clamp is resting on the mucosal surface, while the solid leaf is resting against the skin surface (Figure 2a). The clamp is tightened until superficial vessels are engorged and sublabial glands distended against mucosa (Figure 2b). Care is taken not to over-tighten the clamp, to avoid causing discomfort to the patient.

Fig. 1. (a) Frontal and (b) side views of the chalazion clamp demonstrating the fenestrated and non-fenestrated leaf and adjustment screw.
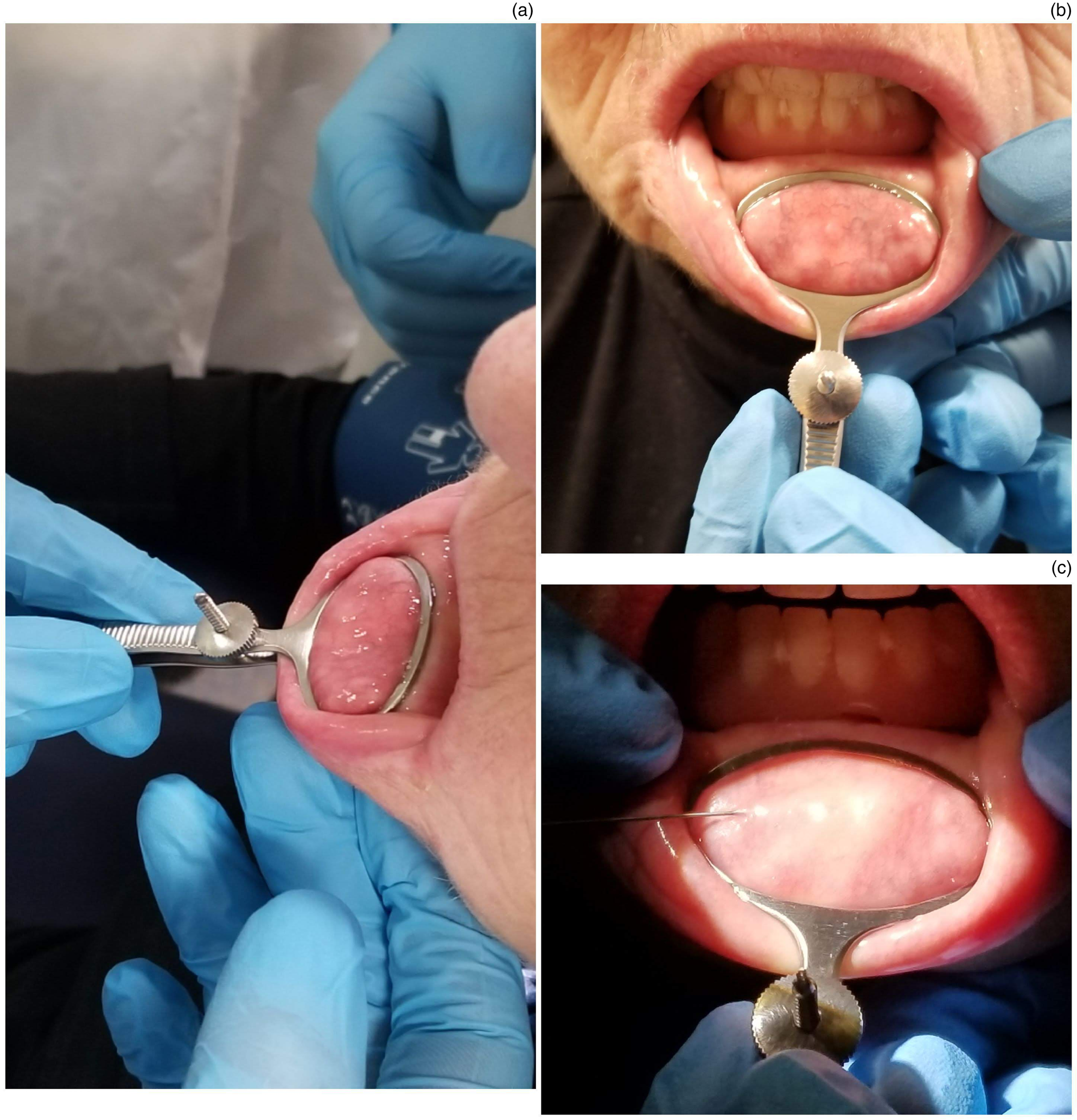
Fig. 2. (a) Side and (b) frontal views of the clamp in situ, and (c) image demonstrating local anaesthetic infiltration with a dental syringe.
Local anaesthetic (1–1.5 ml) is infiltrated, to ensure adequate anaesthesia and to aid haemostasis (Figure 2c). A 2 cm horizontal incision is made until submucosal tissue is exposed (Figure 3a). Some of the submucosal glands may readily extrude from the incision site. These are excised and placed in a formaldehyde specimen jar (Figure 3b). A blunt dissection is carried out, to obtain at least four to five visualised glands in a dry surgical field (Figure 3c). Limited bipolar diathermy can be used to ensure adequate haemostasis, or to prophylactically ensure haemostasis, where severed blood vessels could be readily identified in the field. Prior to this stage, it is good practice to positively identify a branch of the mental nerve (Figure 3d).
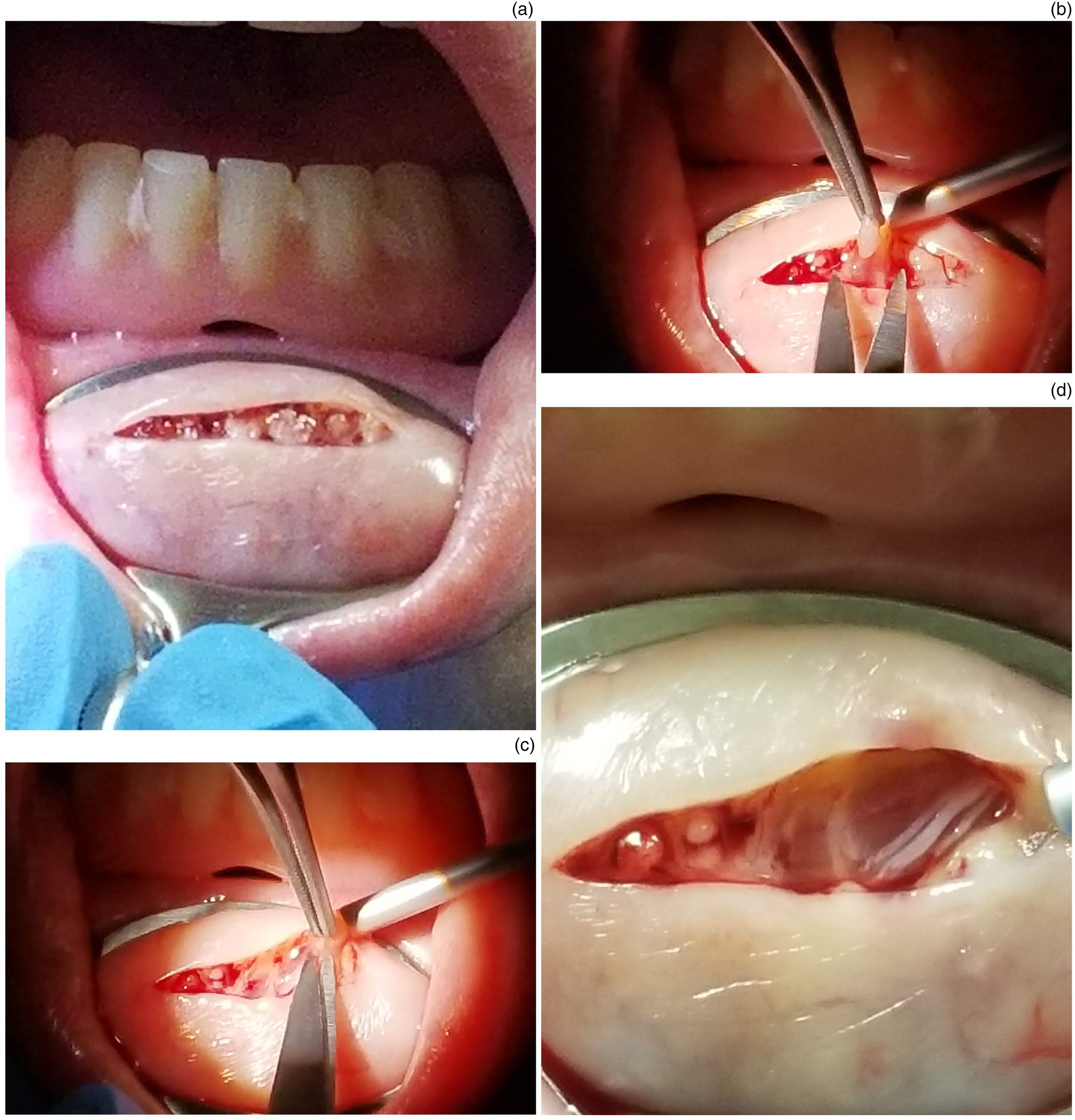
Fig. 3. Images demonstrating: (a) the incision performed, (b) the glandular tissue identified, (c) the dissection of these glands using Adson's forceps and Iris scissors, and (d) the mental nerve carefully preserved.
Results
Forty-five cases were identified. The mean age of patients was 56.1 years, with 3 males and 42 females, and a male-to-female ratio of 1:14. After exclusion criteria were applied, 41 cases remained.
All operators outlined the specific number of glands harvested in the intra-operative record. Of the 41 procedures included, all histopathological reports identified sublingual gland tissue (100 per cent). Histopathological reports identified a specified number of glands, or histology was reported as ‘multiple glands harvested’. Thirty-two specimens (78 per cent) had a specified number of glands harvested, eight (19.5 per cent) were described as having multiple glands present and one (2.5 per cent) did not specify quantity (Figure 4). The number of glands harvested varied from four to nine glands. The median number harvested was 5.5 (interquartile range = 4.75–6).
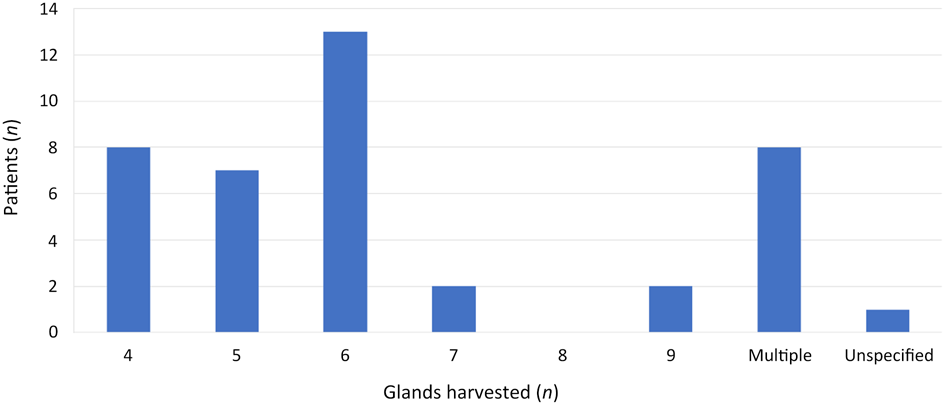
Fig. 4. Summary of numbers of glands harvested, as identified by histopathological analysis.
Twenty-nine per cent of patients (n = 12) were identified as having primary Sjögren's syndrome on histological analysis. Fifty-six per cent (n = 23) were negative for Sjögren's syndrome and 15 per cent (n = 6) had inconclusive findings. Our data demonstrated a sensitivity of 92.31 per cent (95 per cent confidence interval (CI) = 63.97–99.81) and a specificity of 100 per cent (95 per cent CI = 84.56–100.00). The positive predictive value was 100 per cent and the negative predictive value was 95.65 per cent (95 per cent CI = 76.99–99.31). The disease prevalence was 37.14 per cent (95 per cent CI = 21.47–55.08) in this group of patients.
At follow up, no patient (0 per cent) had a complication when reviewed one month later in the out-patients department.
Discussion
Sublingual gland biopsy is an invaluable tool in diagnosing primary Sjögren's syndrome. Patients presenting with xeropthalmia and xerostomia have a wide differential diagnosis, including but not limited to age-related sicca syndrome, immunoglobulin G4 related disease, lymphoepithelial sialadenitis, granulomatous disease and haematological malignancy. Despite the working diagnosis of all patients in our series being primary Sjögren's syndrome, only 29 per cent of patients displayed histological characteristics of the disease. This is an important consideration in the investigation and subsequent management of these patients, and re-affirms the important role for sublabial gland biopsy in their diagnosis. In addition to its use in diagnosing primary Sjögren's syndrome, sublabial gland biopsy can also be helpful in the diagnosis of amyloidosis, sarcoidosis and neonatal haemochromatosis.Reference Varela-Centelles, Seoane-Romero, Sánchez-Sánchez, González-Mosquera, Diz-Dios and Seoane10
The accuracy of this procedure is imperative in order to provide adequate tissue such that mononuclear infiltration can be observed, characterised and differential pathological processes ruled out. Given that this procedure is invasive and not without its complications, one must have confidence in the surgical technique, of which several have been described. Fisher and colleagues’ data, collated from International Collaboration between Rheumatology Research Group colleagues, suggested a minimum of four glands to allow proper histological analysis.Reference Fisher, Jonsson, Daniels, Bombardieri, Brown and Morgan11 The most characteristic feature of primary Sjögren's syndrome on biopsy is focal lymphocytic sialadenitis, which describes the presence of dense aggregates (foci) of 50 or more mononuclear cells (mostly lymphocytes), in a periductal or perivascular localisation.Reference Fisher, Jonsson, Daniels, Bombardieri, Brown and Morgan11
The sensitivity and specificity values of sublabial gland biopsy are variable. A meta-analysis concluded a high diagnostic value for primary Sjögren's syndrome, with specificity of 88 per cent (standard deviation (SD) ± 11.7) and sensitivity of 78.8 per cent (SD ± 11.2), a positive predictive value of 87.6 per cent (SD ± 9.5) and a negative predictive value of 79.0 per cent (SD ± 16.9).Reference Guellec, Cornec, Jousse-Joulin, Marhadour, Marcorelles and Pers12 Our sensitivity and specificity values are amongst the highest reported, with sensitivity of 92 per cent and specificity of 100 per cent observed in the present study.
Despite its utility, there is a notable paucity of standardisation of the sublabial gland biopsy procedure. Earlier studies describe a ‘punch’ technique, but, because of inadequate specimen provision, this method has been largely replaced by a more technically complex but higher yield surgical method.Reference Bertram and Hjgrting-Hansen13 Of these more conventional procedures, several techniques have been described, with marked variations in incision length (0.1–3 cm), incision direction (vertical, circular or oblique) and instruments used.Reference Varela-Centelles, Seoane-Romero, Sánchez-Sánchez, González-Mosquera, Diz-Dios and Seoane10,Reference Colella, Cannavale, Vicidomini and Itro14 One study described a technique that could be used by non-surgical physicians in the out-patient setting.Reference Friedman, Miller and Huszar15
On review of the literature, harvest rates are not elucidated for each individual method, and subsequently no direct comparison has been performed between techniques. Where harvest rates are reported, significant heterogeneity exists. The largest published series of sublabial gland biopsy in Sjögren's syndrome, of 362 patients, reports a miss rate of 10 per cent.Reference Daniels16 More recently, a report from 502 patients with suspected Sjögren's syndrome or amyloidosis described a miss rate of less than 1 per cent.Reference Caporalli, Bonacci, Epis, Bobbio-Pallavincini, Morbini and Montecucco17 This is in contrast to our current study where no insufficient samples were reported.
• Sublabial gland biopsy is important in diagnosing Sjögren's syndrome; four glands are ideally harvested to provide adequate tissue for histopathological review
• Given the harvest site location, the difficulty identifying anatomical structures and the risk to the mental nerve, a dry surgical field is desirable
• Chalazion clamp use can achieve an ischaemic field, facilitating swift and seamless removal of the glands, unobscured by troublesome haemorrhage
• The accuracy of this technique was 100 per cent in our series of 41 patients, with all specimens providing adequate glandular tissue
• No patient in our series suffered a complication, indicating the safety of this procedure
Chalazion clamps are most commonly found in the hands of our ophthalmic surgery colleagues when performing incision and drainage of a chalazion. However, their roles in other specialties have been described for nearly 50 years.Reference Garcia and Davis18 The fenestrated leaf lends itself perfectly to exposing the operating field, while the adjustment screw allows an adjustable tourniquet effect, providing variable degrees of ischaemia to allow intervention. This relative ischaemia is invaluable in the process of identifying the small white, semi-translucent sublabial glands from surrounding connective tissue, where even a minor amount of haemorrhage will obscure subtle macroscopic distinguishing features. In addition to identifying the glands themselves, blood vessels may be managed in a proactive manner; the mental nerve may be identified to minimise the risk of traction or thermal injury, lessening the incidence of resultant hypoaesthesia. In this series, no patient reported numbness of the lower lip, in comparison to the 6–11 per cent rates described in the literature.Reference Caporalli, Bonacci, Epis, Bobbio-Pallavincini, Morbini and Montecucco17
Conclusion
Subsequent to our earlier report in 2019 describing our initial experience with the chalazion clamp, we found that this method of providing a dry surgical field has once again proven to be reliable and safe. In our present study using this technique, there was a 100 per cent harvest rate, with no sample found to be insufficient. Building upon this series going forward in the future, we hope to draw further comparisons between this and other more traditional techniques.
Competing interests
None declared








