INTRODUCTION
Typing of resistant Neisseria gonorrhoeae in conjunction with epidemiological information provided by infected patients aids our understanding of the development and spread of infection with antibiotic-resistant strains. Recently, molecular typing by N. gonorrhoeae multi-antigen sequence typing (NG-MAST) has replaced the more conventional phenotyping methods [Reference Martin1]. NG-MAST is highly discriminatory, produces objective data that is easily shared and can be performed on a large scale. This method is gaining international acceptance as a typing tool for use in the surveillance of gonococcal infection [Reference Herida2–Reference Unemo4] although there is some evidence that sequence types can be further subdivided by opa-typing [Reference Martin1].
Ciprofloxacin resistance in N. gonorrhoeae has continued to rise in the United Kingdom. The most recent data from Scotland reported a significant increase from 19·1% in 2004 to 23·6% in 2005 [Reference Young and Palmer5]. Similarly in England and Wales there was a significant rise in ciprofloxacin-resistant isolates from 14% in 2004 to 21·7% in 2005 [6]. We have previously used NG-MAST to type a collection of 106 gonococcal isolates which demonstrated reduced susceptibility or resistance to ciprofloxacin and were collected from across Scotland during 2002 as part of our continuous surveillance programme [Reference Palmer7]. The aim of this work was to establish if sequence type clusters defined in the previous study could be subdivided by opa-typing and, if so, to determine whether epidemiological data supported any of the subdivisions found.
METHODS
N. gonorrhoeae isolates
All isolates sent to the Scottish Neisseria gonorrhoeae Reference Laboratory (SNGRL) were tested for antibiotic susceptibility by agar plate dilution as previously described [Reference Young8]. Decreased susceptibility to ciprofloxacin was defined as MIC ⩾0·125–0·5 mg/l and ciprofloxacin resistance was defined as MIC ⩾1 mg/l.
A collection of 106 Scottish isolates from 2002 with decreased susceptibility or resistance to ciprofloxacin had previously been typed using NG-MAST [Reference Palmer7]. Of these, 74 had non-unique sequence types and were selected for further subtyping. All isolates were grown on selective New York City agar (bioMérieux, Basingstoke, UK) and incubated overnight at 37°C in 5% CO2 prior to subculture onto non-selective New York City agar and incubation as before.
Opa-typing
A 1 μl loopful of N. gonorrhoeae was suspended in 150 μl 5% Chelex-100 resin (Bio-Rad Laboratories Ltd, Hemel Hempstead, UK) slurry in distilled water. Samples were heated (95°C for 10 min), centrifuged (2 min at 13 000 g) and the supernatant stored at −20°C prior to use. Opa-typing was performed as previously described with minor modifications [Reference Palmer, Leeming and Turner9]. Amplification was carried out in a 50 μl reaction volume containing 1×PCR buffer (Qiagen Ltd, Crawley UK), 200 μm of each dNTP, 0·5 μm of oligonucleotides opa-up 5′-GCGATTATTTCAGAAACATCCG-3′ and opa-down 5′-GCTTCGTGGGTTTTGAAGCG-3′ [Reference O'Rourke10], 2 μl DNA lysate and 1·25 U ‘HotStarTaq’ (Qiagen). After an initial denaturation step of 95°C for 15 min, amplification was performed using 35 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 30 s. The final extension reaction was carried out at 72°C for 10 min. PCR reactions were performed in a GeneAmp PCR system 9600 (Applied Biosystems, Warrington, UK CA, USA). PCR products were digested separately with Taq αI (New England Biolabs Ltd, Hitchin, UK), or HhaI (Promega, Southampton, UK) and analysed by polyacrylamide gel electrophoresis as previously described [Reference Palmer, Leeming and Turner9]. Each isolate was amplified and digested in duplicate. Opa-types were considered to be distinguishable if they differed by two or more bands in Taq αI and HhaI digests.
Epidemiological data
Epidemiological data, routinely collected on all N. gonorrhoeae isolates sent to the SNGRL, were retrieved for each isolate in the study. The statistical significance of any associations between typing and epidemiological characteristics was tested using the χ2 test (http://www.georgetown.edu/faculty/ballc/webtools/web_chi.html).
RESULTS
Opa-typing
Opa-typing of the 74 ciprofloxacin-resistant isolates revealed a total of 20 opa-types (OT). Six isolates had unique opa-types whilst the remaining opa-types were represented by more than one isolate. The largest opa-type cluster (OT 1) contained 21 isolates (see Table 1). Opa restriction digest fragment patterns typically contained 10–15 DNA fragments for each of the enzymes used.
Table 1. Epidemiological data for 32 isolates defined as Opa-types 1–5 and S T147

OT, Opa-type; NG-MAST, Neisseria gonorrhoeae multi-antigen sequence type.
Comparison of NG-MAST and opa-typing
The 74 isolates were divided into 12 sequence types. Seven sequence types (ST), containing 2–4 isolates, were each concordant with a single opa-type: ST 66, ST 84, ST 154, ST 278, ST 307, ST 310 and ST 311. Five sequence types were not concordant with a single opa-type: ST 147 (32 isolates), ST 203 (four isolates), ST 211 (four isolates), ST 304 (seven isolates) and ST 314 (nine isolates). With the exception of ST 147, these sequence types were each divided into two opa-types. The opa-types in ST 203 were subdivided by four band differences, while the remaining three sequence types were subdivided into opa-types by two band differences. ST 147, containing 32 isolates, was subdivided into five opa-types (OT 1–5). OT 1 and OT 2, containing 21 and eight isolates respectively, differed from each other by two bands. OT 3–OT 5, each containing a single isolate, differed from OT 1 by 3–5 band differences.
Ciprofloxacin intermediate isolates
Ten of the 74 isolates selected demonstrated decreased susceptibility to ciprofloxacin. These ten isolates were restricted to three opa-types (OT 10, 15 and 16) corresponding to ST 84, 66, and 154 which contained only isolates of decreased susceptibility to ciprofloxacin.
Epidemiological associations and opa-type
Fifty isolates were from heterosexual individuals, 17 isolates came from men who had sex with men (MSM) and there was no sexual preference data for seven patients. Both OT 1 and OT 2 subdivided ST 147; heterosexual patients accounted for a significantly higher proportion of infections with OT 1 (90%, 18/20) than with OT 2 (29%, 2/7) (χ2=10·2, P=0·0014) (see Table 1).
The majority of infections (80%, 49/61) were acquired through sexual activity in the United Kingdom. For 13 patients there was no data on acquisition. The majority of isolates both in OT 1 (94% 16/17) and OT 2 (100% 6/6) were acquired in the United Kingdom (χ2=0·37, P=0·54) (see Table 1).
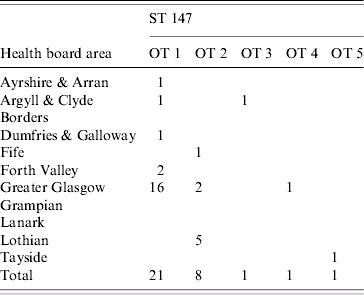
Most isolates were from the major Scottish conurbations of Greater Glasgow and Lothian (principally Edinburgh), 35/74 and 17/74 respectively. There was clustering of opa-types in some areas. OT 1 was found on the west coast of Scotland (Greater Glasgow, Ayrshire & Arran, Argyll & Clyde and Dumfries & Galloway) significantly more often than OT 2 (χ2=12·4, P=0·0004). Both these opa-types formed part of the ST 147 cluster (see Table 2).
Table 2. Geographical distribution of 32 isolates defined as Opa-types (OT) 1–5 (ST 147) classified by health board area
DISCUSSION
The data presented here confirm that opa-typing frequently subdivides NG-MAST clusters. In this study of ciprofloxacin-resistant isolates five of the 12 sequence types could be subdivided by opa-typing compared with eight of the 14 major clusters of isolates from the study in London [Reference Martin1]. In both studies the number of fragment differences between opa-types within any sequence type was extremely low (⩽5) and it is probable that these isolates are still highly related.
However, are the small differences detected by opa-typing indicative of epidemiologically distinct groups of isolates? In the case of four of the five sequence type clusters where opa-typing yields further subdivisions, the number of isolates was too small for meaningful epidemiological analysis (4–9 isolates in each cluster). However, the increased resolution of opa-typing was concordant with epidemiological data for the largest cluster, ST 147 which contained 32 isolates. OT 1 and OT 2, which differed by only two band differences, accounted for 90% (29/32) of the ST 147 isolates. These may have separate epidemiological niches: OT 1 was more common in heterosexuals than was OT 2 (P=0·0014) and OT 1 was also more common in the West of Scotland than in the rest of the country (P=0·0004). This is the first demonstration of concordant epidemiological and opa-type subdivisions within a NG-MAST cluster.
Isolates of the ST 147 genotype were present in the United Kingdom as early as 1999; retrospective typing of representative isolates of the predominant ciprofloxacin-resistant clone circulating amongst heterosexuals in Greater Manchester in 1999 [Reference Palmer, Leeming and Turner9] identified this as ST 147 (H. M. Palmer, unpublished observations). Further isolates of ST 147 have been recorded from London between 2000 and 2003, with a predominantly homosexual route of transmission [Reference Martin11] and in Scotland in 2001–2002 predominantly amongst heterosexuals. Isolates of ST 147 have also been recorded in other countries (Denmark [Reference Hoffman12], United States [Reference Trees13] and France – see www.ng-mast.net). Clearly this strain has become successfully established and appears to have spread to two epidemiological groups with differing sexual preference as early as 2000. It is possible that genetic variation resulting from within these separate niches is reflected in the small differences detected by opa-typing.
The data for the ST 147 cluster demonstrates the value of both the epidemiological information used in conjunction with the NG-MAST data, and the discriminatory power of opa-typing. The routine use of opa-typing on a large scale is not practical due to the subjective nature of fragment pattern analysis, but may be of value where epidemiological data or other biological data (e.g. antibiotic susceptibility patterns) indicate possible heterogeneity within a sequence type cluster. NG-MAST is much more suitable than opa-typing for large-scale surveillance owing to the objective nature of the sequenced-based data and the web-based system of identifying each unique sequence type with a number. In biological terms these data suggest that genetic changes (mutations and/or recombination events) are accumulated in the hypervariable region of the opa genes sampled by opa-typing more rapidly than they are in the regions of the por and tbpB genes sampled by NG-MAST. Nevertheless, NG-MAST remains the primary typing tool for use in any large-scale epidemiological surveillance project. As yet NG-MAST has not been used in any specific public health interventions, but timely provision of such data may make this possible in the future.
ACKNOWLEDGEMENTS
We are grateful to all of our laboratory and clinical colleagues throughout Scotland for submitting isolates to the SNGRL and providing detailed epidemiological information.
DECLARATION OF INTEREST
None.






