The breath test with the use of the 1,3-distearyl-2-([13C]-octanoyl-)glycerol, known also as the 13C-mixed TAG (13C-MTG), was first described by Vantrappen et al. (Reference Vantrappen, Rutgeerts and Ghoos1) and was designed for the purpose of assessing, in a non-invasive way, exocrine pancreatic efficiency. Since then it has been successfully applied to both adults and children, and even in newborns(Reference Vantrappen, Rutgeerts and Ghoos1–Reference Van Dijk-van Aalst, Van Den Driessche and Van der Schoor5). The literature provides evidence that the 13C-MTG breath test is a useful diagnostic tool for patients suffering from diseases such as cystic fibrosis of the pancreas(Reference De Boeck, Delbeke and Eggermont4, Reference Maes, Ghoos and Geypens6), chronic pancreatitis(Reference Vantrappen, Rutgeerts and Ghoos1), coeliac disease(Reference Perri, Pastore and Festa7) or steatorrhoea(Reference Iglesias-García, Vilariño-Insua and Iglesias-Rey8). It has also been applied to check the efficiency of supplementary therapy with pancreatic enzymes(Reference De Boeck, Delbeke and Eggermont4, Reference Amarri, Harding and Coward9, Reference Herzog, Delvin and Albert10), and to evaluate and monitor exocrine pancreatic function in patients undergoing pancreatic resections(Reference Mrowiec, Lampe and Kasicka-Jonderko11–Reference Nakamura, Morifuji and Murakami13). Recently, this test has been applied for the purpose of assessing the impact of a weight-reducing pharmacotherapy with orlistat upon intra-intestinal lipolytic activity(Reference Kocełak, Zahorska-Markiewicz and Jonderko14).
According to reports published to date, the composition and/or the energy content of the test meals applied while performing the 13C-MTG breath test differed greatly, depending on the particular research group. The common feature is that the meals usually consisted of bread and butter. For example, Vantrappen et al. (Reference Vantrappen, Rutgeerts and Ghoos1) in their pioneering work used a test meal consisting of 100 g toasted bread, and butter to the amount of 0·25 g/kg of body mass. The same test meal composition was used by another team(Reference Perri, Pastore and Festa7), which was examining patients suffering from coeliac disease. Nakamura et al. (Reference Nakamura, Morifuji and Murakami13) used 90 g toasted bread with 15 g margarine and 200 ml milk to assess exocrine pancreatic function in patients after pancreatic surgery. They proved that the 13C-MTG breath test may be more efficient diagnostically than the measurement of faecal elastase-1 concentration. Boedeker et al. (Reference Boedeker, Goetze and Pfaffenbach15) conducted research on healthy volunteers and patients suffering from chronic pancreatitis and exocrine pancreatic malfunction. They were given 100 g white bread, 20 g butter and 250 ml coffee with no milk or sugar. A Belgian team chose a test meal of a pancake containing 18 g fat, 18 g carbohydrates and 12 g protein(Reference De Boeck, Delbeke and Eggermont4). The Domínguez-Muñoz research group administered 13C-MTG with 20 g butter, two slices of toasted white bread and 200 ml water to drink(Reference Domínguez-Muñoz, Iglesias-García and Igesias-Rey16, Reference Domínguez-Muñoz, Iglesias-García and Vilariño-Insua17). Mexican researchers administered 13C-MTG with 15 g butter, 90 g bread and an additional 240 ml chamomile tea to drink(Reference Móran-Villota, Artega and Rodríguez-Leal18).
More complicated preparations of the test meal have also been reported. Löser et al. (Reference Löser, Brauer and Aygen19) homogenised the 13C-labelled substrate with 10 g chocolate spread at 60°C in a water bath, and after cooling, they administered it to the examined persons with one slice of toasted bread, spread with 15 g butter, and allowed them to drink 200 ml water. Another research team(Reference Van Dijk-van Aalst, Van Den Driessche and Van der Schoor5) administered 13C-MTG with 15 g chocolate spread, 5 g butter, a slice of white bread and 100 ml milk to drink. Schneider et al. (Reference Schneider, Hammerstingl and Heller20) gave their volunteers 30 g chocolate spread with two 50 g slices of toast and 200 ml water. Finally, it should be mentioned that in investigations conducted in infants, the test meal was composed of NAN1 modified milk, wherein 13C-MTG was suspended(Reference Van Dijk-van Aalst, Van Den Driessche and Van der Schoor5).
It seems therefore necessary to conduct systematic research on the optimisation of the composition of the carrier test meal, with which the 13C-labelled substrate is to be administered. Of interest is whether it would be feasible to reduce the energy content of the test meal, specifically by lowering the amount of the unlabelled fat given together with the 13C-MTG. Accordingly, the goal of the research was to elaborate the conduct of the 13C-MTG breath test ensuring simple handling of the substrate and convenience for the patient, while at the same time preserving optimum 13CO2 elimination kinetics in the exhaled air.
Materials and methods
A total of eighteen healthy volunteers (ten women and eight men) were examined. The average age of the volunteers was 26·5 (se 1·4) years and the BMI was 22·36 (se 0·62) kg/m2.
During a screening interview, the participants declared being in good health according to WHO criteria(21). Exclusion criteria for participation in the study comprised: malnutrition and/or symptoms suggestive of impaired absorptive function of the gut, history of surgery affecting the anatomy of the digestive tract (except for an appendectomy) and pregnancy. None of the subjects took any medication known to influence gastric emptying or intestinal transit, or which could affect exocrine pancreatic function.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the bioethics committee of the Medical University of Silesia. Informed, written consent was obtained from all subjects. During an introductory interview, the subjects were instructed not to eat any food naturally rich in 13C, such as products containing maize, cane sugar, pineapple or kiwi fruit for the 48 h preceding the examination(Reference Kasicka-Jonderko, Jonderko and Kamińska22, Reference Jonderko, Kasicka-Jonderko and Kamińska23).
Study protocol
The subjects reported to the laboratory in the morning after a 12-h overnight fast, during which cigarette smokers (two subjects) were additionally asked to refrain from smoking.
The 13C-MTG breath test was carried out four times in every subject on separate days. The average interval between consecutive examinations was 8 d (median, interquartile range: 5–21 d). The female volunteers were always examined in the same phase of their menstrual cycle(Reference Jonderko24).
At the beginning of every examination, a basal fasted sample of the exhaled air was taken, so as to determine the referential 13CO2 enrichment. Next, the subjects were given in random order:
(1) 300 mg 13C-MTG containing 99 % of 13C (code INC650P; Euriso-Top S.A., Saint-Aubin, France) in two wafers = MTG.
(2) 300 mg 13C-MTG in two wafers and a 50 g wheat roll = MTG-WR. The wheat roll was purchased from a local supplier and was baked from wheat flour type 550 in compliance with the Polish norm PN-92/A-74 105(25). According to the Food Composition Data Base of the National Food and Nutrition Institute in Warsaw(26), a 50 g wheat roll provides 571 kJ and contains 4·1 g protein, 28·9 g carbohydrates, 0·8 g fat and 0·9 fibre (product code: 6·4·3·003).
(3) 50 g wheat roll spread with 10 g butter and with 300 mg 13C-MTG = MTG-WR-10B. According to the Food Composition Data Base, a 100 g aliquot of the butter type ‘Extra’ (product code: 5·2·1·001) contains 3075 kJ, 82·5 g fat, 0·7 g protein and 0·7 g carbohydrates(26). Hence the WR-10B meal provided 879 kJ from 4·1 g protein, 28·9 g carbohydrates, 9·0 g fat and 0·9 fibre.
(4) 50 g wheat roll spread with 30 g butter and with 300 mg 13C-MTG = MTG-WR-30B. This meal had a total of 1494 kJ and contained 4·3 g protein, 20·1 g carbohydrates, 25·5 g fat and 0·9 fibre.
In addition, for reasons of comfort, on each occasion the volunteers were allowed to drink 200 ml black tea without sugar after completion of any of the four varieties of 13C-MTG administration.
After ingestion of the substrate/test meal, samples of the exhaled air were taken every 30 min for 6 h, counting from the ‘0’ time, defined as the moment of the application of the substrate. The procedure of collecting breath air was standardised: the volunteers took a breath and held it for 10 s, then steadily blew the air through a straw of 3 mm inner diameter into a special glass tube of 12 ml capacity (Exetainer®; Labco Limited, High Wycombe, UK), that was then immediately tightened with a dedicated plastic cap(Reference Kasicka-Jonderko, Jonderko and Kamińska27). During the study, the volunteers did not take any other food or drink, and remained in a comfortable sitting position watching films.
13CO2 enrichment in the samples was subsequently measured by means of MS with the use of an Automated Breath 13C Analyser device (Ser-Con Limited, Crewe, UK). The quality control procedure involved a run of the measurement on a standard gas (5 % CO2 within N2) of a certified 13CO2 content of − 31·33 ‰ before and after every series of breath air samples(Reference Kasicka-Jonderko, Loska and Jonderko28). The 13CO2 content determined within the total pool of the exhaled CO2 was expressed in Δ‰ PDB units, i.e. in accordance with the international standard, which is the calcium carbonate of the fossil Belemnitella of the cretaceous Pee Dee formation in South Carolina, USA (zero Δ‰ PDB corresponds to 1·11 123 13C atoms in CaCO3)(Reference Craig29). The net changes in 13CO2 concentration were conveyed as Δ over baseline (DOB) units according to the following formula:
where ‘i’ represents the probe number and ‘0’ pertains to the basal probe of expiratory air.
Following the procedures described previously(Reference Jonderko, Duś and Szymszal30), curves of the momentary and cumulative recovery of 13C in the exhaled air relative to the administered oral dose of 13C-MTG were constructed within the time domain of 0–6 h. The following parameters were then derived: D max, the maximum momentary 13C recovery, and T max, the time elapsing from the substrate application to the D max occurrence, as well as the cumulative 13C recovery, AUC, defined as the percentage of the administered substrate dose eliminated with the exhaled air within 6 h.
Statistical analysis
Taking into account our former research on reproducibility of the 13C-MTG breath test(Reference Kasicka-Jonderko, Jonderko and Szostak31), the sample size of n 18 was chosen. According to the results obtained, with a within-subject study protocol involving paired examinations taken at an interval of 16–22 d, a sample of twelve subjects shows the smallest detectable difference in AUC amounting to 3·48 % dose (at the P = 0·05 level, two-tailed). Augmentation of the sample size to 18 would be expected to enable detection of a difference of 2·72 % dose.
The results were subjected to repeated-measures ANOVA(Reference Armitage32). The differences between means were checked post hoc with Tukey's honest significant difference test(Reference Armitage32). Statistical significance was set at the P < 0·05 level, two-tailed. The results are presented as means with their standard errors. All statistical analyses were performed using Statistica 6.0 software (StatSoft, Inc., Tulsa, OK, USA)(33).
Results
The basal fasted concentration of 13CO2 in the exhaled air amounted to − 27·33 (se 0·28) Δ‰ PDB on the day of MTG, − 27·12 (se 0·24) Δ‰ PDB on the day of MTG-WR, − 27·12 (se 0·28) Δ‰ PDB on the day of MTG-WR-10B and − 27·20 (se 0·26) Δ‰ PDB on the day of MTG-WR-30B. The repeated-measures ANOVA indicated that those initial concentrations of 13CO2 did not differ among particular research sessions (F 3;27 = 0·321, P = 0·81).
After an oral intake of 300 mg 13C-MTG only, a statistically significant increase in the concentration of 13CO2 in the exhaled air was observed during the interval between the 120th and 300th min, whereas after the consumption of 13C-MTG-WR a statistically significant increase in the content of 13CO2 in the exhaled air occurred between the 180th and 360th min of observation. After administration of 300 mg 13C-MTG with the 50 g wheat roll and 10 g butter, a pronounced increase in the concentration of 13CO2 in the exhaled air was noted as early as after 60 min and it remained statistically significant until the end of the observation. An increase in the amount of the unlabelled fat to 30 g caused a 30-min delay in the occurrence of the statistically significant increase above the baseline of the concentration of 13CO2 in the exhaled air.
Fig. 1 represents the time course of the curves of momentary 13C recovery in the exhaled air. Intake of the sole 13C-MTG in a wafer resulted in a low and flat course of the curve. In the case of the application of 13C-MTG with the 50 g wheat roll but without the addition of unlabelled fat, there was a clear peak of the group curve of the momentary 13C recovery at the 270th min that, however, did not exceed 4·0 % dose/h (Fig. 1). Consumption of 13C-MTG together with 10 or 30 g unlabelled fat enabled the achievement of much better time courses of the curves of momentary 13C recovery in the exhaled air. A look at the respective curves plotted in Fig. 1 suggests a shift towards the right of the curve of momentary 13C recovery after the consumption of MTG-WR-30B compared with the situation with MTG-WR-10B. The maximum of the group curve of 13C recovery after the application of MTG-WR-10B, amounting to 7·36 (se 0·63) % dose/h, was observed at the 210th min of observation, whereas the peak of the group curve of the momentary 13C recovery after the application of MTG-WR-30B of 7·12 (se 0·60) % dose/h occurred 60 min later (Fig. 1).

Fig. 1 Momentary 13C recovery in breath air after the consumption of 300 mg 13C-mixed TAG (300 mg 13C-MTG; ▲), 300 mg 13C-MTG and a 50 g wheat roll (****), a 50 g wheat roll spread with 10 g butter and with 300 mg 13C-MTG (●), a 50 g wheat roll spread with 30 g butter and with 300 mg 13C-MTG (■).
Table 1 shows the group averages of the parameters characterising quantitatively the 13CO2 elimination kinetics in breath air. These data suggest that, compared to the consumption of sole 13C-MTG, the application of this substrate with a carbohydrate meal (50 g wheat roll) did not significantly improve the maximum momentary 13C recovery, but in fact caused a significant delay in the occurrence of the D max – the difference between the corresponding mean T max values amounted to 72 min and was statistically significant.
Table 1 Kinetics of elimination of 13CO2 in the expiratory air after intake of 300 mg 13C-mixed TAG (MTG), 300 mg 13C-MTG and a 50 g wheat roll (MTG-WR), a 50 g wheat roll spread with 10 g butter and 300 mg 13C-MTG (MTG-WR-10B), a 50 g wheat roll spread with 30 g butter and 300 mg 13C-MTG (MTG-WR-30B)
(Mean values with their standard errors)
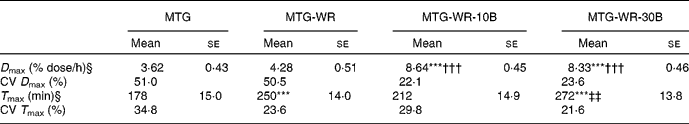
*** Mean values were significantly different from those of MTG (P < 0·001).
††† Mean values were significantly different from those of MTG-WR (P < 0·001).
‡‡ Mean values were significantly different from those of MTG-WR-10B (P < 0·01).
§ Parameters of the breath test: D max, momentary 13C recovery; T max, time of occurrence of the D max.
The application of 13C-MTG with the addition of unlabelled fat resulted in a more than double increase in the average maximum 13C recovery in expiratory air, compared with the situation after the intake of the sole substrate administered in a wafer. Differences observed in the D max after the application of either the MTG-WR-10B or the MTG-WR-30B, when related to the reference situation with the intake of the sole 13C-MTG, were statistically highly significant (Table 1). It should also be mentioned that the addition of 10 or 30 g unlabelled fat contributed to more than a twofold diminution of the CV of the D max, when compared to the situation after the administration of the sole 13C-MTG or the substrate together with a carbohydrate meal (Table 1).
The increase of the dose of the unlabelled fat from 10 to 30 g did not augment the maximum momentary 13C recovery, but caused a 60-min delay in the occurrence of the D max, and the respective difference in the T max was statistically significant (Table 1).
Fig. 2 displays the time course of the curves of the cumulative 13C recovery in the exhaled air. After the intake of the sole 13C-MTG in a wafer, the cumulative 13C recovery in the exhaled air was unsatisfactory and did not exceed 10 % dose after 6 h. The application of 13C-MTG with the carbohydrate meal (50 g wheat roll) did not improve this parameter, which was proven by the statistical comparisons presented in Table 2. At each time interval, for which the AUC was calculated, there were no statistically significant differences between the MTG and the MTG-WR. At the same time, the time course of the cumulative 13C recovery curves plotted in Fig. 2, as well as the results of the respective statistical comparisons presented in Table 2, indicate that the addition of the unlabelled fat contributed to a dramatic improvement in the 13C recovery in the exhaled air. Paradoxically, the addition of a smaller amount of butter allowed us to obtain higher values of the cumulative 13C recovery in the exhaled air – statistically significant differences between the MTG-WR-10B and the MTG-WR-30B were found for the AUC calculated within a time interval ranging from 0–60 to 0–270 min (Table 2).

Fig. 2 Cumulative 13C recovery in breath air after the consumption of 300 mg 13C-mixed TAG (300 mg 13C-MTG; ▲), 300 mg 13C-MTG and a 50 g wheat roll (◆), a 50 g wheat roll spread with 10 g butter and with 300 mg 13C-MTG (●), a 50 g wheat roll spread with 30 g butter and with 300 mg 13C-MTG (■).
Table 2 Cumulative 13C recovery in breath air (AUC) after the intake of 300 mg 13C-mixed TAG (MTG), 300 mg 13C-MTG and a 50 g wheat roll (MTG-WR), a 50 g wheat roll spread with 10 g butter and 300 mg 13C-MTG (MTG-WR-10B), a 50 g wheat roll spread with 30 g butter and 300 mg 13C-MTG (MTG-WR-30B)
(Mean values and standard errors)

a,b,c Mean values with unlike superscript letters were significantly different from those of MTG, MTG-WR, and MTG-WR-30B, respectively.
Discussion
The results of the present research show that the composition of the applied meal, with which the 13C-labelled substrate enters the digestive tract, has a significant impact on the 13C elimination kinetics in breath air. Hoping for a possible simplification of the procedure of administering the 13C-labelled substrate to subjects, which would be very helpful under clinical circumstances, we decided to check on the performance of the breath test with the sole 13C-MTG, placed in two starch capsules. It turned out, however, that the curves of the 13C recovery in the exhaled air appeared to be low and flat. Hence an unsatisfactory cumulative 13C recovery of < 10 % dose within 6 h was obtained. Administration of 13C-MTG with a carbohydrate test meal as a carrier (50 g wheat roll) resulted in only a slight and statistically insignificant increase in the maximum momentary 13C recovery in the exhaled air, which occurred on average 72 min later.
Such a shift of T max can be referred to the kinetics of the emptying of the test meal from the stomach. Knowledgeably the gastric evacuation of a solid food comprises a so-called lag phase that corresponds to the time necessary to crumble the solids into parts not bigger than 1–2 mm, and only thereafter a proper linear evacuation follows(Reference Siegel, Urbain and Adler34–Reference Jonderko35). Nonetheless, in the case of the test meal of a 50 g wheat roll taken as the 13C-MTG carrier, the values of the cumulative 13C recovery remained unsatisfactory – the AUC after 6 h amounted on average to 11·5 % dose. It is noteworthy that, either after administration of the sole 13C-MTG or after its application with a 50 g wheat roll, quite a high inter-individual variability of the D max was observed (compare with the respective CV included in Table 1).
The results of this study provide proof that the addition of unlabelled fat contributes to a dramatic improvement in the 13C recovery in the exhaled air. One of the possible explanations for this phenomenon may be the more efficient stimulation of the gallbladder contraction exerted by the fat load. Knowledgeably, the release of cholecystokinin from the I cells of the small-intestrial mucosa is stimulated in the first place by the products of fat digestion(Reference Degen, Matzinger and Drewe36, Reference McLaughlin, Lomax and Hall37). One can suppose that in the case of the 50 g wheat roll test meal, containing predominantly complex carbohydrates, the emptying of the gallbladder might be too small to deliver an amount of bile into the intestine lumen sufficient for the digestion of the 13C-MTG. Admittedly, this explanation should be considered as a hypothesis because we did not measure gallbladder-emptying nor plasma-cholecystokinin profiles. Nevertheless other authors also point to the importance of fat-stimulated emptying of the gallbladder in obtaining a desired time course of the curves of 13C elimination in breath air after per oral 13C-MTG administration(Reference Hernell38, Reference Keller39).
An important issue is the amount of unlabelled fat that should be consumed to perform the 13C-MTG breath test properly. The short review of the test meals applied by various research groups to date, provided earlier in the paper, clearly indicates that nowadays there does not exist any standardisation in this respect. According to an editorial comment published recently in Digestion, an increased fat load could be expected to improve the sensitivity and specificity of the 13C-MTG breath test, but no more specific recommendations were given therein(Reference Keller39). In the present study, we found more favourable values of the parameters of breath 13C elimination kinetics after per oral administration of 13C-MTG with 10 g butter, when compared with the situation after the consumption of 30 g butter. In particular, the peak momentary 13C recovery in the expiratory air occurred on average 1 h earlier in the case of the application of the smaller amount of the unlabelled fat, whereas, at the same time, a higher cumulative 13C recovery during the 6-h observation was attained. The results obtained are consistent with the observations of Sonko et al. (Reference Sonko, Prentice and Coward40) who found an inverse relationship between the amount of fat taken orally and its fraction undergoing oxidation within 8 h. Specifically, according to the paper cited, in the case of the consumption of 20 g maize oil the average oxidised fraction amounted to 38·2 %, while after the consumption of 35 g oil the oxidised fraction was on average 32·5 %. It seems that the kinetics of stomach evacuation is a factor that should be taken into account to interpret the results of both research studies – the present study and the one published by Sonko et al. (Reference Sonko, Prentice and Coward40). It should be remembered that the pace of stomach evacuation depends largely on the physical and chemical properties of the consumed meal and is controlled by a number of fine regulatory mechanisms, involving, among others, the duodenal receptors, gastrointestinal hormones and neuromediators. Maes et al. (Reference Maes, Ghoos and Geypens6) carried out an important experiment with the use of two labelled substrates at the same time. They applied 13C-octanoic acid for the evaluation of the kinetics of stomach emptying and 14C-MTG for the assessment of intra-intestinal lipolytic activity. After analysing the results obtained, the authors suggested that a delayed stomach evacuation might disturb intra-intestinal lipolysis, which in turn would have an impact upon the kinetics of 14C recovery in the exhaled air(Reference Maes, Ghoos and Geypens6). At this point, one should mention an item connected to the performance of the 13C-MTG breath test, which has not yet been addressed, namely the problem of potential fat layering within the stomach. Nevertheless, according to the pertinent literature data, the impact of fat layering onto the overall gastric emptying would be expected to be of minor significance, provided that the fat was incorporated within the solid phase of a test meal(Reference Jian, Vigneron and Najean41, Reference Kunz, Feinle-Bisset and Faas42).
To sum up, the present study results indicate, first, that the addition of unlabelled fat is indispensable in obtaining desired values of the parameters characterising breath 13C elimination in the course of the 13C-MTG test. Second, we have found that it is possible to apply a considerably smaller amount of the unlabelled fat with the test meal, compared to the procedures described to date. The test meal examined in this study, consisting simply of a 50 g wheat roll and 10 g butter, seems recommendable for routine use when performing the 13C-MTG breath test because it fulfils a number of essential requirements. A test meal should be easy to prepare from common and continuously available products and it should be representative of a normal daily diet. It should also taste and look attractive to guarantee a favourable reception among examined patients. A drawback of such a composition of the test meal consists in its unsuitability for examinations in gluten-intolerant patients. Hence, the elaboration of a test meal applicable to that particular group of patients may be indicated as a goal for future research work. Accordingly, a prospective study should encompass research on whether it would be possible to resign from the carbohydrate carrier, and a determination of the lowest possible dose of the unlabelled fat while conducting the 13C-MTG breath test.
Acknowledgements
Financial support for the project was provided by the Medical University of Silesia (contract no. KNW-2-108/10). None of the authors has any commercial relationship that might pose a conflict of interest with regard to this paper. M. B. performed the research, accomplished the measurements of the breath samples for 13CO2 enrichment and co-wrote the paper with K. J. who designed the research and analysed the data. M. P. participated in the research and collected the data.








