Pre-eclampsia and other hypertensive disorders of pregnancy occur in 5–10 % of pregnancies and are associated with almost 30 000 maternal deaths, 416 000 stillbirths and 1·5–2 million neonatal deaths annually worldwide(Reference von Dadelszen and Magee1–5). In addition, pre-eclampsia is associated with long-term adverse outcomes including high risk of future CVD, diabetes, dyslipidaemia and chronic kidney disease for the mother and higher risk for attention deficit/hyperactivity disorder, increased BMI and CVD among children exposed to pre-eclampsia(Reference Goffin, Derraik and Groom6–Reference Turbeville and Sasser10). Clinical and social risk factors for pre-eclampsia include prior pre-eclampsia, chronic hypertension, chronic kidney disease, obesity, primiparity, multifetal pregnancy, antiphospholipid antibody syndrome, conception by means of assisted reproductive technology, low socio-economic status and minority ethnic background(Reference Bartsch, Medcalf and Park11–Reference Magee, Nicolaides and von Dadelszen13). The development of pre-eclampsia involves inadequate placentation, maternal inflammatory response, generalised endothelial dysfunction and high blood pressure(Reference Magee, Nicolaides and von Dadelszen13,Reference von Dadelszen, Ayres de Campos, Barivalala, Magee, von Dadelszen and Stones14) . Because nutrition is important for placentation and certain micronutrients have clinical antioxidant, anti-inflammatory and blood pressure regulating properties, maternal nutritional factors may play protective roles or heighten risk of developing pre-eclampsia(Reference Achamrah and Ditisheim15–Reference Poston, Igosheva and Mistry18).
Nutritional factors are acknowledged as a key component in the 2016 WHO recommendations on routine antenatal care (ANC) for promoting maternal and child health and fourteen of the forty-nine recommendations relate to nutrition in pregnancy(19). For pre-eclampsia prevention specifically in the WHO ANC and pre-eclampsia guidelines, nutritional interventions are limited to high dose (1·5–2 g daily) Ca supplementation in populations with low Ca dietary intake(19,20) . A review of clinical practice guidelines for pregnancy hypertension found that only aspirin and Ca were commonly recommended for the prevention of pre-eclampsia(Reference Scott, Gillon and Pels21). The tendency to focus on single nutrients obscures the complexity of possible interactions and causal pathways(Reference Hiatt, Porco and Liu22), which may be important to understand given the complex nature of pre-eclampsia. The multi-factored evolution of risk with maternal nutrition and other clinical, biologic, social and environmental factors is not well understood, and this impacts capacities for developing prevention strategies.
This evidence review aims to compile definite, probable, possible and indirect nutritional determinants of pre-eclampsia reported in current literature, by magnitude of effect and quality of evidence in order to map a nutritional conceptual framework for pre-eclampsia prevention.
Methods
We followed the methods of Hiatt et al.(Reference Hiatt, Porco and Liu22) to develop a model of determinants using a systematic process. First, a broad group of pre-eclampsia experts were selected from the Epidemiology Working Group of the PREgnancy Care Integrating translational Science, Everywhere (PRECISE) Network to develop components for a working model of pre-eclampsia determinants divided into medical history, biomarkers, nutrition and social determinants quadrants(Reference Magee, Strang and Li23). Each of the quadrants was independently investigated and refined through a literature review to confirm associations, expand indicators and evaluate evidence. The present study focuses on the diet and nutrition quadrant.
Search strategy
The diet and nutrition literature review involved a systematic search on the Cochrane Library and Medline Ovid from database inception to 11 October 2022, on Google Scholar and reference lists. Searches were conducted using the following terms: (pre-eclampsia OR preeclampsia) AND (pregnant OR pregnancy) AND (deficiency OR deficient OR nutrient OR nutrition OR supplement OR status).
The highest level of evidence supporting associations between risk factors and pre-eclampsia was identified in a hierarchical manner based on Grading of Recommendations, Assessment, Development and Evaluation (GRADE) standards(Reference Balshem, Helfand and Schünemann24). We first sought umbrella reviews (systematic reviews of systematic reviews) reporting on nutritional factors and pre-eclampsia. If no relevant umbrella reviews were identified, then the process was expanded to identify relevant meta-analyses. High-quality meta-analyses, such as Cochrane systematic reviews, were prioritised where available. The process was repeated with individual randomised controlled trials (RCT), then large observational studies. We included observational studies with at least 1000 participants to attempt to be more representative of the general population and higher likelihood of sufficient statistical power to assess specific determinants(Reference Bartsch, Medcalf and Park11,Reference Sterne, Gavaghan and Egger25) . We excluded smaller observational studies, case reports or series, qualitative reviews and editorials. Articles not written in English were excluded due to limited capacity of the review team to comprehensively search non-English databases.
Study selection
Titles and abstracts of articles were screened to assess their eligibility based on study design (umbrella review, meta-analysis, RCT or large observational study), population (pregnant or women of reproductive age), exposure (nutritional biomarker or dietary pattern) or intervention (nutritional supplement or dietary intervention) and outcome (pre-eclampsia or known risk factor for pre-eclampsia). Potentially eligible studies reporting quantitative direct or indirect associations between nutritional factors and pre-eclampsia underwent full-text review. Articles were initially screened by MWK and then discussed with the British Columbia PRECISE Conceptual Framework Working Group (KP, SP, OC) for final decision on inclusion.
Data extraction
Author, year, publication type (umbrella review, systematic review/meta-analysis, RCT, observational), risk factor, outcome, study design, number of participants, relative effect (95 % CI), variation between studies (I 2), strength of association and quality of evidence were extracted from each study onto a standardised, piloted data extraction form on Word (Microsoft Corporation). Relative effects of nutritional factors were extracted as relative risks (RR), OR, standardised mean difference (SMD) or calculated from the prevalence of pre-eclampsia (or known risk factor of pre-eclampsia for indirect associations) among women with and without the risk factor. Study characteristics necessary to assess evidence quality were also extracted. Data were extracted by MWK and quality checked by members of the British Columbia PRECISE Conceptual Framework Working Group (KP, SP, OC).
Strength of association and quality of evidence assessment
Larger magnitude of effects is indicative of stronger evidence that the risk factor has an impact on the outcome and strength of association was assessed as definite (RR < 0·40 or ≥3·00), probable (RR 0·40–0·69 or 1·50–2·99), possible (RR 0·70–0·89 or 1·10–1·49) or not discernible/not significant (RR 0·90–1·09)(Reference Hiatt, Porco and Liu22,Reference Colditz, Atwood and Emmons26,Reference Jaeschke, Guyatt and Sackett27) . Because pre-eclampsia occurs in less than 10 % of the exposed and unexposed populations, OR are a reasonable approximation of the RR and used interchangeably for the model(Reference Viera28).
Quality of evidence was evaluated using GRADE and classified as high, moderate, low or very low(Reference Balshem, Helfand and Schünemann24). Umbrella reviews, systematic reviews and RCT started as high certainty of evidence, while observational studies started as low certainty of evidence(Reference Balshem, Helfand and Schünemann24). Studies were downgraded for potential risk for bias, inconsistency, indirectness, imprecision and publication bias and upgraded for large effect sizes and evidence of a dose–response(Reference Balshem, Helfand and Schünemann24). Potential publication bias was indicated with an asymmetrical funnel plot(Reference Balshem, Helfand and Schünemann24,Reference Higgins, Thomas and Chandler29) . Studies could be down or upgraded by one or two levels depending of the severity within each domain(Reference Balshem, Helfand and Schünemann24).
Extracted data and GRADE evaluations were reviewed within the University of British Columbia PRECISE Conceptual Framework Working Group (MWK, KP, SP, OC) with oversight from nutrition experts (RE, SEM, HDM) and clinical experts (LAM, PvD) to ensure validity. Discrepancies were discussed until consensus was achieved. The model was refined based on input from the PRECISE Conceptual Framework Working Group. Nutritional factors were cross-checked with patient interests raised in The Preeclampsia Registry(Reference Tsigas30). Priority areas raised by pre-eclampsia patients and families included folic acid, Fe, Na, vitamin D, Ca, fish oil and Mg.
Results
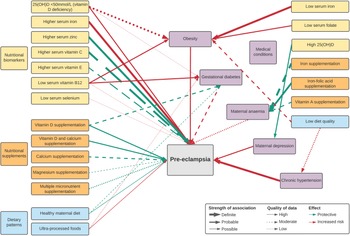
Overall, twenty-five nutritional factors were reported in two umbrella reviews(Reference Giannakou, Evangelou and Papatheodorou12,Reference Kinshella, Omar and Scherbinsky31) and twenty-two meta-analyses(Reference Song, Luo and Zhang32–Reference Paula, Patriota and Gonçalves54). These included eight biomarker levels (25(OH)D, Fe, Zn, Cu, Se, vitamins C, E and B12), fourteen nutritional supplementations (Ca and/or vitamin D, vitamin C and/or E, vitamin B6, Fe and/or folic acid, Mg, Zn, multiple micronutrients, n-3 fatty acids, balanced protein and energy), one dietary intervention (antenatal dietary counselling) and two dietary patterns (healthy maternal dietary pattern, ultra-processed foods). Fourteen factors were significantly associated with pre-eclampsia incidence (Table 1) while evidence did not support a significant association for eleven factors (online Supplementary Table S1). Additionally, there were fifteen nutritional factors potentially indirectly associated with pre-eclampsia incidence (Table 2) based on an umbrella review(Reference Griffith, Alsweiler and Moore55), fifteen meta-analyses(Reference Palacios, Kostiuk and Pena-Rosas37,Reference Kibret, Chojenta and Gresham39,Reference Pena-Rosas, De-Regil and Garcia-Casal44,Reference Middleton, Gomersall and Gould47,Reference Paula, Patriota and Gonçalves54,Reference McCauley, van den Broek and Dou56–Reference Tan, Liu and Chen65) and three large cohort studies(Reference Scholing, Olthof and Jonker66–Reference Yee, Silver and Haas68). A summary of associations is illustrated in Fig. 1.
Table 1. Nutritional factors with significant associations with risk of developing pre-eclampsia (95 % confidence intervals)

RR, relative risk; WMD, weighted mean difference; SMD, standardised mean difference; NA, not applicable.
* Protective against developing pre-eclampsia.
Table 2. Nutritional factors with potential indirect associations with pre-eclampsia incidence via medical conditions (95 % confidence intervals)

RR, relative risk.
* Protective effects.
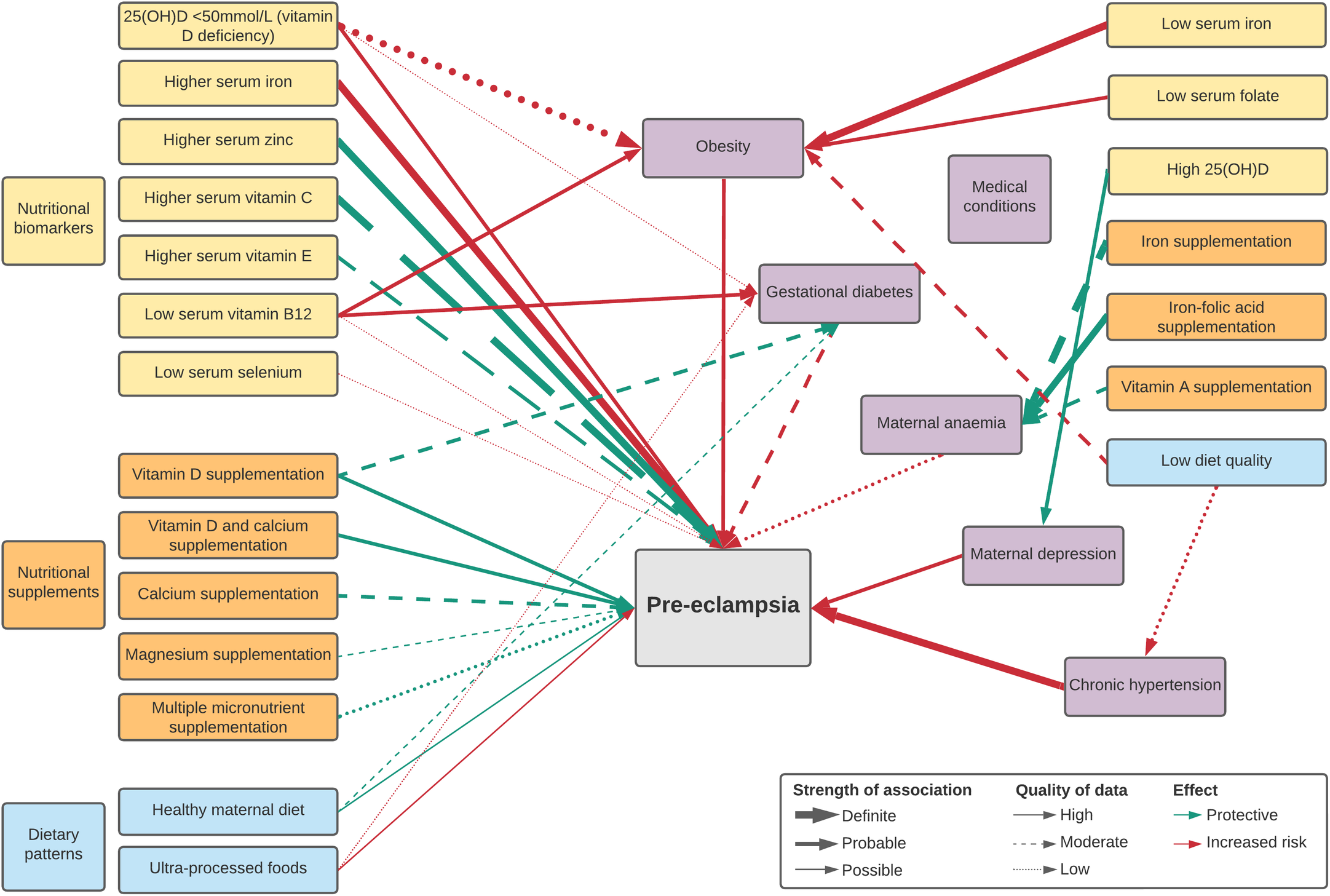
Fig. 1. Map of significant direct and indirect nutritional risk factors for pre-eclampsia.
Definite associations
There were three nutritional factors with definite associations (Table 1). Higher serum Fe status was a risk factor while higher serum Zn was protective, based on high-quality evidence, and higher serum vitamin C was protective, based on moderate-quality evidence. High heterogeneity between studies was reported.
Women with pre-eclampsia had significantly higher serum Fe concentrations compared with healthy pregnant controls (SMD 1·27, 95 % CI (0·76, 1·78), 1912 participants, twenty-three studies, I 2 96 %), largely assessed in the third trimester with high heterogeneity that remained after sensitivity analyses(Reference Song, Luo and Zhang32). An umbrella review subsequently assessed that higher maternal serum Fe had almost ten times the odds of developing pre-eclampsia(Reference Giannakou, Evangelou and Papatheodorou12). Increased maternal serum Fe levels among pre-eclamptic women were confirmed in another meta-analysis, which found higher levels among both Asian and European populations(Reference Liu, Chen and Li69).
Women with pre-eclampsia had lower Zn concentrations compared with healthy pregnant controls (SMD − 0·587, 95 % CI (−0·963, −0·212), 1091 participants, fourteen studies, I 2 88 %), measured largely in the third trimester(Reference Ma, Shen and Zhang33). Significantly lower Zn concentrations among pre-eclamptic women compared with healthy controls were only found in Asia(Reference Ma, Shen and Zhang33,Reference Zhu, Zhang and Chen70,Reference Jin, Hu and Zheng71) and sub-Saharan Africa(Reference Tesfa, Nibret and Munshea72), not in other regions of the world.
Third-trimester concentrations of maternal serum vitamin C were significantly lower among women with pre-eclampsia (SMD − 0·56, 95 % CI (−0·83, −0·28), 2777 participants, twenty-nine studies, I 2 91 %)(Reference Cohen, Beddaoui and Kramer34). Evidence for serum vitamin C had moderate certainty of evidence, due to the inclusion of some low-quality studies in meta-analyses and potential publication bias.
Probable associations
There were six probable associations (Table 1). Protective effects of vitamin D on its own or co-supplemented with Ca were reported with high-quality evidence, maternal serum vitamin E status and Ca supplementation with moderate-quality evidence and maternal multiple micronutrient supplementation with low-quality evidence. Maternal vitamin D deficiency was a risk factor with high-quality evidence. High heterogeneity between studies was found for both Ca supplementation and serum vitamin E.
Maternal vitamin D deficiency, indicated by 25(OH)D < 50 mmol/l, was associated with increased odds of developing pre-eclampsia(Reference Giannakou, Evangelou and Papatheodorou12,Reference Wei, Qi and Luo35) . A larger effect of vitamin D deficiency compared with insufficiency (<75 mmol/l) suggests a potential dose–response, though confidence intervals overlap (deficiency OR 2·11, 95 % CI (1·52, 2·94) v. insufficiency OR 1·72, 95 % CI (1·11, 2·69))(Reference Giannakou, Evangelou and Papatheodorou12,Reference Wei, Qi and Luo35) . A subsequent meta-analysis with more included studies (twenty-one studies, 39 031 participants) also found a significant effect of vitamin D deficiency when measured around the second trimester among all populations except Oceanic groups(Reference Yuan, Tai and Xu73).
Third-trimester serum vitamin E was significantly lower among women with pre-eclampsia (SMD − 0·42, 95% CI (−0·72, −0·13), 3398 participants, thirty-four studies, I 2 93 %)(Reference Cohen, Beddaoui and Kramer34). A recent large multicentre Chinese cohort study with 73 317 women found that low first-trimester serum vitamin E < 7·3 mg/l was also associated with higher risk of developing pre-eclampsia(Reference Shi, Jiang and Yuan74). Moderate certainty of evidence resulted from the inclusion of some low-quality studies and potential publication bias.
Protective effects of vitamin D and Ca supplementation, each on their own, or supplemented together have been well documented in an umbrella review(Reference Kinshella, Omar and Scherbinsky31), Cochrane systematic reviews(Reference Palacios, Kostiuk and Pena-Rosas37,Reference Hofmeyr, Lawrie and Atallah38) and a network meta-analysis(Reference Woo Kinshella, Sarr and Sandhu52). The majority of studies were conducted in low- and middle-income countries (LMIC); sensitivity analyses excluding high-income countries did not significantly change effects(Reference Kinshella, Omar and Scherbinsky31). An earlier review of Ca supplementation in LMIC also found a significantly reduced risk of pre-eclampsia(Reference Imdad, Jabeen and Bhutta75). Higher dosages of vitamin D during pregnancy did not significantly increase benefit in comparison with lower dosages on the risk of developing pre-eclampsia (>600 mg/d v. ≤600 mg/d, ≥40 000 mg/d v. <40 000 mg/d), nor did commencement of supplementation either before or after 20 weeks gestation, though evidence was limited(Reference Palacios, Kostiuk and Pena-Rosas37,Reference Palacios, Trak-Fellermeier and Martinez76) . Both high (≥1 g/d) and low dose (<1 g/d) Ca supplementation had evidence of a strong beneficial effect, while Ca supplementation commencing early around the periconceptual period was not significant, based on very low-quality evidence. Ca supplementation overall had moderate certainty of evidence due to heterogeneity and potential publication bias.
A probable association between multiple micronutrient supplementation and pre-eclampsia prevention is based on low quality of evidence. Only two studies were found to report pre-eclampsia as an outcome, neither study using the United Nations standardised multiple micronutrients formulation(Reference Kinshella, Omar and Scherbinsky31). While both included studies a significant beneficial effect, the selection criteria of study populations, timing and content of supplementation were different.
Possible associations
There were five possible factors associated with pre-eclampsia prevention (Table 1). Lower maternal serum vitamin B12 (Reference Mardali, Fatahi and Alinaghizadeh36) and Se(Reference Hamdan, Hamdan and Adam51) may heighten risk for the development of pre-eclampsia, based on low certainty of evidence. Serum vitamin B12 was on average 15·24 pg/mL lower among women with pre-eclampsia when compared with those without(Reference Mardali, Fatahi and Alinaghizadeh36). Significantly lower Se concentrations among pre-eclamptic women compared with healthy controls were only found in African-based studies(Reference Hamdan, Hamdan and Adam51). Mg supplementation may lower the odds of developing pre-eclampsia, based on moderate certainty of evidence. Pooled outcomes found a significant beneficial effect, though many of the individual Mg trials had non-significant results(Reference Yuan, Yu and Zhu53).
Based on high-quality evidence, a healthy maternal dietary pattern characterised by high intake of fruits, vegetables, whole-grain foods, fish and poultry as highlighted in Mediterranean and New Nordic diets was associated with 22 % reduced odds of developing pre-eclampsia(Reference Kibret, Chojenta and Gresham39). The review consisted of four, large, high-income country-based cohort studies: three from the Norwegian Mother and Child Cohort Study (MoBa) that assessed maternal diet in the second trimester(Reference Brantsæter, Haugen and Samuelsen77–Reference Torjusen, Brantsæter and Haugen79) and the Generation R Cohort Study from the Netherland with assessment at a median of 13·5 weeks(Reference Timmermans, Steegers-Theunissen and Vujkovic80). A subsequent meta-analysis of LMIC-based studies found that adequate (≥1–3 servings/week) vegetable consumption reduced the odds of developing pre-eclampsia by 62 % (OR 0·38, 95 % CI (0·18, 0·80), four studies, 1391 participants, I 2 85 %) and by 58 % with adequate (≥1–3 servings/week) fruit consumption (OR 0·42, 95 % CI (0·24, 0·71), five studies, 1676 participants, I 2 79 %) compared with women with low or no consumption(Reference Kinshella, Omar and Scherbinsky81). Conversely, maternal diets characterised by ultra-processed foods were associated with higher odds of developing pre-eclampsia, based on high-quality evidence and no heterogeneity between study results(Reference Paula, Patriota and Gonçalves54).
Not discernible
Based on moderate-quality evidence, antenatal dietary counselling was not significantly associated with pre-eclampsia prevention(Reference Kinshella, Omar and Scherbinsky31) (online Supplementary Table S1). According to our methodology, there was no evidence supporting a direct association between maternal serum Cu(Reference Giannakou, Evangelou and Papatheodorou12,Reference Fan, Kang and Zhang40) or supplementation with any antioxidants(Reference Rumbold, Duley and Crowther41), vitamin B6 (Reference Salam, Zuberi and Bhutta49), vitamin C and/or E(Reference Kinshella, Omar and Scherbinsky31,Reference Rumbold, Ota and Nagata42,Reference Rumbold, Ota and Hori43) , Fe and/or folic acid(Reference Kinshella, Omar and Scherbinsky31,Reference Pena-Rosas, De-Regil and Garcia-Casal44) , Zn(Reference Kinshella, Omar and Scherbinsky31,Reference Mori, Ota and Middleton46) , n-3 fatty acids(Reference Kinshella, Omar and Scherbinsky31,Reference Middleton, Gomersall and Gould47) or protein-energy addition(Reference Ota, Hori and Mori48) and pre-eclampsia prevention, all based on low to very-low quality evidence. See online Supplementary Table S2 for the GRADE assessment of each nutritional factor.
Indirect associations
Nutritional factors with potential indirect associations with pre-eclampsia incidence via medical conditions are reported in Table 2. These include maternal anaemia (Hb <11 g/dl)(Reference Young, Oaks and Tandon82), particularly in the first trimester(Reference Jung, Rahman and Rahman83) and when severe (Hb <7 g/dl)(Reference Bilano, Ota and Ganchimeg84), gestational diabetes mellitus (GDM)(Reference Bilano, Ota and Ganchimeg84), maternal overweight (BMI 25·0–29·9)(Reference Bartsch, Medcalf and Park11,Reference Townsend, Khalil and Premakumar85) and obesity (BMI ≥ 30)(Reference Bartsch, Medcalf and Park11,Reference Giannakou, Evangelou and Papatheodorou12,Reference Townsend, Khalil and Premakumar85) , antenatal depression(Reference Hu, Li and Zhang86) and chronic hypertension (pre-existing or hypertension diagnosed before 20 weeks)(Reference Bartsch, Medcalf and Park11,Reference Townsend, Khalil and Premakumar85) (see also online Supplementary Table S3).
Maternal anaemia may be lowered by Fe–folic acid supplementation and Fe supplementation based on high and moderate quality of evidence(Reference Pena-Rosas, De-Regil and Garcia-Casal44), and possibly by vitamin A supplementation based on moderate quality of evidence(Reference McCauley, van den Broek and Dou56). There was no evidence for an effect of folic acid(Reference Lassi, Salam and Haider57), multiple micronutrient (any formulation, compared with Fe with or without folic acid)(Reference Keats, Haider and Tam58), Ca(Reference Buppasiri, Lumbiganon and Thinkhamrop59) or n-3 fatty acids(Reference Middleton, Gomersall and Gould47) supplementation (see online Supplementary Table S1).
Four nutritional factors were associated with risk of GDM. Based on high-quality evidence, low serum vitamin B12 increased risk of GDM(Reference Kouroglou, Anagnostis and Daponte61). Evidence around vitamin D is strengthened by corresponding findings that low maternal 25(OH)D increased risk of GDM(Reference Tripathi, Rao and Pandey60) while vitamin D supplementation was protective(Reference Palacios, Kostiuk and Pena-Rosas37,Reference Griffith, Alsweiler and Moore55) . A healthy maternal dietary pattern may reduce GDM rates(Reference Kibret, Chojenta and Gresham39), while conversely dietary patterns rich in ultra-processed foods may increase GDM rates(Reference Paula, Patriota and Gonçalves54). Vitamin D and Ca co-supplementation(Reference Palacios, Kostiuk and Pena-Rosas37,Reference Griffith, Alsweiler and Moore55) , n-3 supplementation(Reference Middleton, Gomersall and Gould47,Reference Griffith, Alsweiler and Moore55) and antenatal dietary counselling(Reference Griffith, Alsweiler and Moore55,Reference Tieu, Mcphee and Crowther62) were not associated with rates of GDM.
Obesity was associated with five nutritional factors. Based on high-quality evidence, low serum Fe at 12–15 weeks gestation had a definite association with obesity, while low serum vitamin B12 and serum folate had moderate associations(Reference Scholing, Olthof and Jonker66). Low 25(OH)D (as defined by individual study authors for vitamin D deficiency) was a strong risk factor associated with over three-fold increased odds of obesity, but based on low-quality evidence due to high heterogeneity, potential publication bias and unclear quality of included studies(Reference Yao, Zhu and He63). Poor maternal diet quality (lowest tertile v. highest tertile on the Diet Quality Index for Pregnancy at 26–28 weeks gestation) had a probable association with obesity based on moderate-quality evidence(Reference Laraia, Bodnar and Siega-Riz67). Dietary diversity (among adult men and women)(Reference Salehi-Abargouei, Akbari and Bellissimo64) and maternal serum ferritin(Reference Scholing, Olthof and Jonker66) were not associated with obesity.
Based on high-quality evidence, women with the highest concentrations of maternal 25(OH)D significantly reduced the odds of antenatal and/or postnatal depression compared with women in the lowest category(Reference Tan, Liu and Chen65). A large observational cohort study found that maternal low diet quality (lowest tertile v. highest quartile on the Healthy Eating Index) had a probable association with increased chronic hypertension(Reference Laraia, Bodnar and Siega-Riz67). No other nutritional factors for maternal depression and chronic hypertension were found according to our methodology.
Discussion
Summary of findings
Based on the magnitude of effect and evidence quality (online Supplementary Table S4), higher serum Fe was a strong nutritional risk factors for pre-eclampsia incidence across populations. Low serum Zn was a risk factor particularly in Asia and Africa, but Zn supplementation trials did not reduce pre-eclampsia incidence. Similarly, while there was some evidence of a protective effect of adequate maternal vitamin C and E, supplementation trials did not significantly reduce rates of pre-eclampsia.
Ca supplementation was by far the most studied nutrient in clinical trials to prevent pre-eclampsia, though high heterogeneity between study findings and potential publication bias led to an overall moderate certainty of evidence. Vitamin D supplementation with or without Ca tended to be investigated more recently. Though fewer trials than with Ca, vitamin D supplementation had higher certainty of evidence, with low heterogeneity and less potential publication bias. Certainty of the evidence is supported by complementary findings that vitamin D deficiency is associated with increased risk, while vitamin D supplementation reduced risk of developing pre-eclampsia, both supported by high-quality evidence. Vitamin D may also be indirectly associated with lower pre-eclampsia incidence through its protective effects on GDM, obesity and maternal depression.
Healthy maternal dietary patterns were possibly associated with lower risk of developing pre-eclampsia, with strong evidence from large, longitudinal studies, and evidence of larger effects in LMIC where malnutrition is prevalent. High-quality maternal diets were also protective against GDM, while low-quality diets increased risk of obesity and chronic hypertension. Evidence of healthy maternal dietary patterns is reinforced by increased risk of diet characterised by ultra-processed foods.
There was weak evidence for vitamin B12 deficiency as a risk factor, potentially through increased risk of GDM and obesity. Evidence was also limited for maternal Se levels and multiple micronutrient supplementation. Our evidence review did not find a significant association between overall antenatal dietary counselling and reduced risk of pre-eclampsia.
Comparisons with existing literature and implications for practice
Previous umbrella reviews on pre-eclampsia determinants were not focused on maternal nutritional factors and/or only considered risk factors reported in systematic reviews with direct associations with pre-eclampsia(Reference Giannakou, Evangelou and Papatheodorou12,Reference Kinshella, Omar and Scherbinsky31,Reference Townsend, Khalil and Premakumar85) . The current review includes potential indirect pathways through medical conditions, particularly obesity, maternal anaemia and GDM. For example, maternal deficiencies in vitamin D, B12, folate and Fe were associated with obesity(Reference Yao, Zhu and He63,Reference Scholing, Olthof and Jonker66) , which was related to almost triple the risk of developing pre-eclampsia (RR 2·8, 95 % CI (2·6, 3·1))(Reference Bartsch, Medcalf and Park11). Whether maternal obesity increased the likelihood of nutritional deficiencies, nutritional deficiencies contributed to obesity, or both exacerbated each other remains unclear and methods to disentangle these complex relationships require high-quality data, often collected with longitudinal cohorts (over long periods of time) to assess(Reference VanderWeele87). Amplified by poor quality diets, obesity could be linked to reduced kidney function, altered metabolic processes and gut microbiota, thus preventing adequate nutrient absorption(Reference García, Long and Rosado88,Reference Astrup and Bügel89) . This fits with the Nutritional Conceptual Framework first outlined by the United Nation’s Children’s Fund (UNICEF) in 1990, which emphasised both the lack of adequate, nutritious food alongside frequent illness that impacted the capacity to absorb and utilise nutrients(90,91) . The discrepancies between low serum Zn, vitamin C and E as risk factors for developing pre-eclampsia, yet with the lack of evidence supporting their supplementation, suggest outstanding questions on absorption, utilisation, as well as the timing and dosage of supplementation.
Alongside Ca supplementation, which is recommended by WHO ANC and pre-eclampsia/eclampsia prevention and treatment guidelines(19,20) , Fe, vitamin D and overall healthy maternal diets were other maternal dietary factors that emerged strongly in our evidence review. High and low serum Fe were indicated as direct and indirect risk factors for pre-eclampsia, and recent reviews suggest non-linear relationship where both high and low Hb concentrations were associated with higher pre-eclampsia rates(Reference Young, Oaks and Tandon82,Reference Jung, Rahman and Rahman83) . The WHO currently recommends Fe–folic acid supplementation for all pregnancies (antenatal multiple micronutrient supplements with Fe and folic acid in the context of research). However, understanding potential impacts on pre-eclampsia prevention is challenged by measurement gaps, which focus on perinatal outcomes(Reference Kinshella, Moore and Elango92). For example, a Cochrane review on daily oral Fe supplementation in pregnancy found only four studies that reported pre-eclampsia in comparison with 11 reporting on low birth weight and 13 reporting on preterm birth(Reference Pena-Rosas, De-Regil and Garcia-Casal44).While higher serum Fe was strongly associated with pre-eclampsia, findings largely result from hospital-based, case-controlled studies(Reference Song, Luo and Zhang32). More research in women based studies is needed(Reference Kinshella, Moore and Elango92) to investigate the conditions that lead to pathologically high Fe in women, whether genetic, environmental and/or potentially an effect exacerbated by pre-eclampsia.
A network meta-analysis found that vitamin D may be the best supplementation for lowering pre-eclampsia incidence(Reference Khaing, Vallibhakara and Tantrakul93). In addition to supporting Ca absorption and regulation of blood pressure, vitamin D also has important roles in placental development and inflammation regulation(Reference Olmos-Ortiz, Avila and Durand-Carbajal94). Our review highlights potential indirect associations through GDM, obesity and maternal depression. Further quantification of the extent to which these mediated pathways explain the effect may be useful for further policy development and targeted interventions(Reference Vanderweele95). The WHO ANC guideline recently re-examined vitamin D supplementation, which noted 50 % reductions in the risk of pre-eclampsia and GDM. Supplementation was not recommended, in favour of instead promoting sunlight exposure and adequate nutrition(96).
While our findings that healthy maternal dietary patterns contribute directly and indirectly to pre-eclampsia prevention support the WHO ANC guidelines on promoting healthy maternal diets, the feasibility for women to follow recommendations is a concern, particularly in LMIC(Reference Kinshella, Omar and Scherbinsky31). It is noteworthy that a significant association between antenatal dietary counselling and pre-eclampsia prevention was not found in our review. In contrast, a beneficial effect was found in a previous meta-analysis of six high-income country-based studies of formal dietary counselling(Reference Allen, Rogozinska and Sivarajasingam97), often facilitated by dietitians(Reference Crowther, Hiller and Moss98–Reference Thornton, Smarkola and Kopacz101). A review found that older, more educated women with higher incomes consistently scored higher on diet quality scores during pregnancy across different populations and settings(Reference Doyle, Borrmann and Grosser102), which underscores the importance of socio-economic factors. Immediate causes of malnutrition are influenced by underlying household resources and socio-cultural, economic and political contexts in the UNICEF Nutritional Conceptual Framework(90,91) . With the focus on nutritional education during routine ANC, access to nutritious foods may be a barrier. Impact of nutritional interventions may be limited without a lifelong lens on improving nutrition of girls throughout their lives.
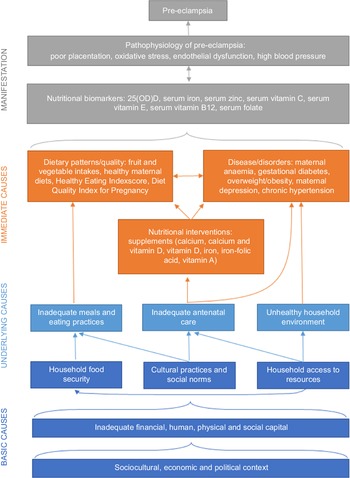
Nutritional conceptual framework for pre-eclampsia and future directions in research
A working conceptual framework to understand dietary risk factors for pre-eclampsia adapted from the UNICEF Nutrition Conceptual Framework (Fig. 2) may help to showcase current gaps and guide future directions in research. There is a need to strengthen evidence on the relationships between nutritional factors, medical conditions and absorption, as well as associations between nutritional factors and underlying/basic causes. Framing nutritional factors by household capacities and socio-cultural, economic and political contexts may shed light on the underlying baseline risks that modify the efficacy of micronutrient supplementation trials, which has largely resulted in few significant effects with the exception of Ca and vitamin D(Reference Kinshella, Omar and Scherbinsky31). With growing understanding that increasing nutritional levels may only be effective in pre-eclampsia prevention when baseline levels are low(Reference Woo Kinshella, Sarr and Sandhu52,Reference Shi, Jiang and Yuan74) , there is a need for future reviews to describe differences between high-income countries and LMIC more explicitly and future research in resource-limited settings to further tease out the impact of underlying risk factors.
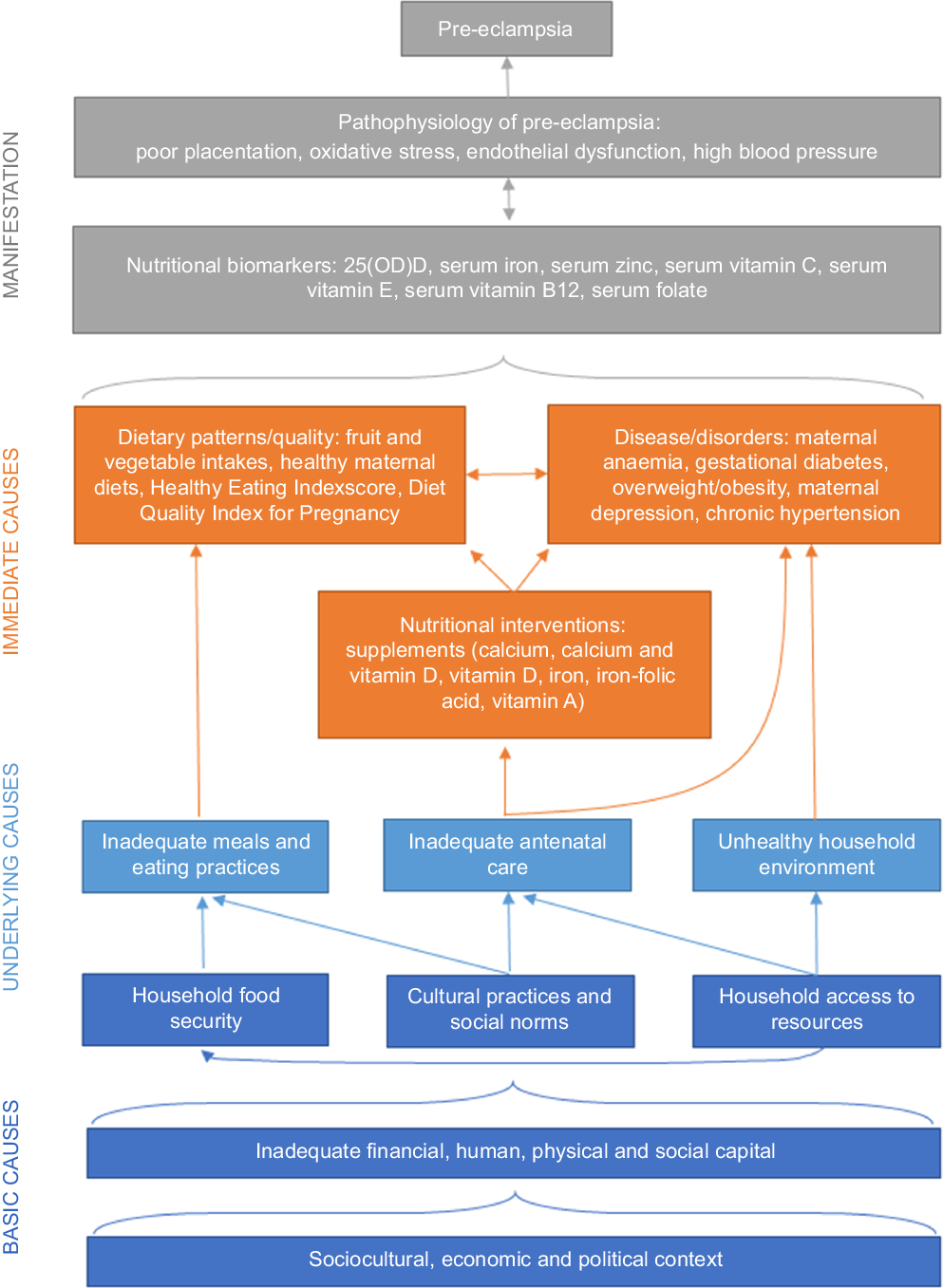
Fig. 2. Nutritional conceptual framework for pre-eclampsia.
Strengths and limitations
Strengths of our analysis include consultation of nutrition and pre-eclampsia experts to guide the development and refinement of variables alongside a systematic methodology following Hiatt et al.(Reference Hiatt, Porco and Liu22) and GRADE standards(Reference Balshem, Helfand and Schünemann24) to compile and critically appraise evidence. Prioritisation of umbrella reviews and Cochrane systematic reviews supported a wide coverage of available studies globally, with rigorous evaluation of their potential risk of bias.
While our evidence review had several quality assurance mechanisms, some limitations included exclusion of non-English studies and lack of double extraction. Additionally, evaluating evidence was challenged by differing capacities to investigate variables. Nutritional biomarkers can be evaluated using objective blood tests, which lends to more certainty of the evidence but are limited by the small panel of biomarkers that researchers select to assess. Supplementation and antenatal counselling interventions are impacted by implementation quality and scope. Dietary patterns and social determinants are often limited by self-reported data, differing definitions between studies and many exposures are not feasible or ethical to evaluate using RCT. Our exclusion of observational studies with less than 1000 participants may have missed some variables. For example, investigating nutritional determinants of obesity among women of reproductive age was challenged by the lack of large cohort studies on the topic. Non-significant results may be inconclusive as there was a lack of high-quality evidence. Compiling evidence around nutritional factors may benefit from more standardised definitions for exposures, outcomes and statistical analyses particularly in observational studies.
Conclusion
Vitamin D, Ca and Fe are strong nutritional factors, both directly and indirectly involved in pre-eclampsia prevention. Healthy maternal diet is a promising approach but more research is needed to understand how best to promote such diets, especially in resource-constrained settings. Zn, vitamins C, E and B are potential areas warranting further investigation, particularly in deficient populations, and around timing of intervention during placental development. A more comprehensive assessment of a full range of nutritional biomarkers is required in future research. We recommend a two-pronged approach: first, to investigate underlying social factors that influence food accessibility and dietary choice and second, to understand nutrient absorption and the impact of co-morbidities, including obesity, GDM and maternal anaemia, as potential mediating factors between maternal dietary intake and risk of developing pre-eclampsia. While WHO guidelines acknowledge the importance of maternal diets for the well-being of mothers and children, nutritional recommendations for pre-eclampsia prevention are currently limited. Recommendations can be strengthened with further evidence-based research into a number of promising areas.
Acknowledgements
The present study is part of the PRECISE (PREgnancy Care Integrating translational Science, Everywhere) Network. The authors would like to express their gratitude to the PRECISE Team for their support. The PRECISE Conceptual Framework Working Group includes King’s College London (Peter von Dadelszen, Laura A. Magee, Lucilla Poston, Hiten D. Mistry, Marie-Laure Volvert, Cristina Escalona Lopez, Sophie Moore, Rachel Tribe, Andrew Shennan, Tatiana Salis-bury, Lucy Chappell, Rachel Craik); Aga Khan University, Nairobi (Marleen Temmerman, Angela Koech Etyang, Sikolia Wanyonyi, Geoffrey Omuse, Patricia Okiro, Grace Mwashigadi); Centro de Investigação de Saúde de Manhiça (Esperança Sevene, Helena Boene, Corssino Tchavana, Euse-bio Macete, Carla Carillho, Lazaro Quimice, Sonia Maculuve); Donna Russell Consulting (Donna Russell); Imperial College London (Ben Baratt); London School of Hygiene and Tropical Medicine (Joy Lawn, Hannah Blencowe, Veronique Filippi, Matt Silver); Midlands State University (Prestige Tatenda Makanga, Liberty Makacha, Yolisa Dube, Newton Nyapwere, Reason Mlambo); MRC Unit The Gambia at LSHTM (Umberto D’Alessandro, Anna Roca, Melisa Martinez-Alvarez, Ha-wanatu Jah, Brahima Diallo, Abdul Karim Sesay, Fatima Touray, Abdoulie Sillah); University of Oxford (Alison Noble, Aris Papageorghiou); St George’s, University of London (Judith Cart-wright; Guy Whitley, Sanjeev Krishna, Rosemarie Townsend, Asma Khalil); University of British Colombia (Marianne Vidler, Joel Singer, Jing (Larry) Li, Jeffrey Bone, Mai-Lei (Maggie) Woo Kinshella, Kelly Pickerill, Ash Sandhu, Domena Tu, Rajavel Elango); University of Malawi (William Stones).
The PRECISE Network was funded by the UK Research and Innovation Grand Challenges Research Fund GROW Award scheme (grant no. MR/P027938/1). MWK was supported by the Vanier Canada Graduate Scholarship funded by the Government of Canada through the Canadian Institutes of Health Research (CIHR) and Canadian Institute of Health Research (FRN 10321) to R.E.
M. W. K. conceptualised the manuscript and developed the methodology with K. P., J. B., M. V., R. C., M. L. V., H. D. M., L. A. M., P. V. D., S. E. M. and R. E. M. W. K., K. P., S. P., O. C. and M. V. contributed to the investigation and analysis, with supervision from R. E., S. E. M., H. D. M., E. T., L. A. M., P. V. D. M. W. K. wrote the initial draft, with all authors involved in review and editing. All authors have read and agreed to the final version of the manuscript.
The authors declare that they have no competing interests.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114522003889