Article contents
Dietary nano-Se supplementation regulates lipid deposition, protein synthesis and muscle fibre formation in grass carp fed with high-fat diet
Published online by Cambridge University Press: 31 March 2023
Abstract
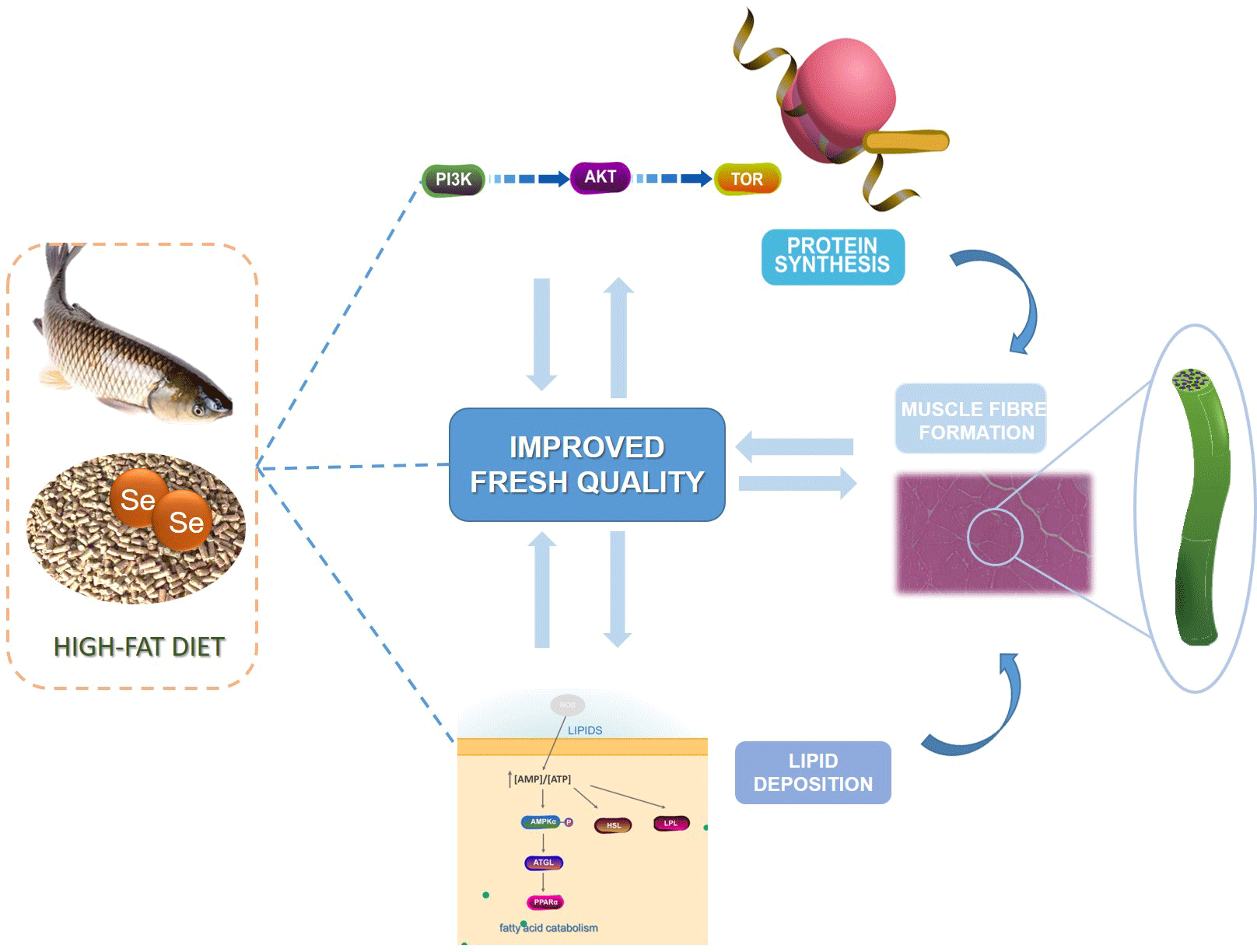
The current study aims to confirm the positive effects of dietary nano-Se on nutrients deposition and muscle fibre formation in grass carp fed with high-fat diet (HFD) before overwintering and to reveal its possible molecular mechanism. The lipid deposition, protein synthesis and muscle fibre formation in grass carp fed with regular diet (RD), HFD or HFD supplemented with nano-Se (0·3 or 0·6 mg/kg) for 60 d were tested. Results show that nano-Se significantly reduced lipid content, dripping loss and fibre diameter (P < 0·05), but increased protein content, post-mortem pH24 h and muscle fibre density (P < 0·05) in muscle of grass carp fed with HFD. Notably, dietary nano-Se decreased lipid deposition in the muscle by regulating amp-activated protein kinase activity and increased protein synthesis and fibre formation in muscle by activating target of rapamycin and myogenic determining factors pathways. In summary, dietary nano-Se can regulate the nutrients deposition and muscle fibre formation in grass carp fed with HFD, which exhibit potential benefit for improving flesh quality of grass carp fed with HFD.
- Type
- Research Article
- Information
- Copyright
- © The Author(s), 2023. Published by Cambridge University Press on behalf of The Nutrition Society
References
- 1
- Cited by





