Book contents
- Frontmatter
- Dedication
- Contents
- List of Contributors
- Editor’s note on the Foreword to the third edition
- Foreword to the third edition
- Foreword to the second edition
- Foreword to the first edition
- Preface
- Acknowledgments
- List of acronyms
- Introduction
- Section I Skeletal trauma
- Chapter 1 The skeleton: structure, growth and development, and basis of skeletal injury
- Chapter 2 Skeletal trauma: general considerations
- Chapter 3 Lower extremity trauma
- Chapter 4 Upper extremity trauma
- Chapter 5 Bony thoracic trauma
- Chapter 6 Dating fractures
- Chapter 7 Differential diagnosis I: diseases, dysplasias, and syndromes
- Chapter 8 Differential diagnosis II: disorders of calcium and phosphorus metabolism
- Chapter 9 Differential diagnosis III: osteogenesis imperfecta
- Chapter 10 Differential diagnosis IV: accidental trauma
- Chapter 11 Differential diagnosis V: obstetric trauma
- Chapter 12 Differential diagnosis VI: normal variants
- Chapter 13 Evidence-based radiology and child abuse
- Chapter 14 Skeletal imaging strategies
- Chapter 15 Postmortem skeletal imaging
- Section II Abusive head and spinal trauma
- Section III Visceral trauma and miscellaneous abuse and neglect
- Section IV Diagnostic imaging of abuse in societal context
- Section V Technical considerations and dosimetry
- Index
- References
Chapter 1 - The skeleton: structure, growth and development, and basis of skeletal injury
from Section I - Skeletal trauma
Published online by Cambridge University Press: 05 September 2015
- Frontmatter
- Dedication
- Contents
- List of Contributors
- Editor’s note on the Foreword to the third edition
- Foreword to the third edition
- Foreword to the second edition
- Foreword to the first edition
- Preface
- Acknowledgments
- List of acronyms
- Introduction
- Section I Skeletal trauma
- Chapter 1 The skeleton: structure, growth and development, and basis of skeletal injury
- Chapter 2 Skeletal trauma: general considerations
- Chapter 3 Lower extremity trauma
- Chapter 4 Upper extremity trauma
- Chapter 5 Bony thoracic trauma
- Chapter 6 Dating fractures
- Chapter 7 Differential diagnosis I: diseases, dysplasias, and syndromes
- Chapter 8 Differential diagnosis II: disorders of calcium and phosphorus metabolism
- Chapter 9 Differential diagnosis III: osteogenesis imperfecta
- Chapter 10 Differential diagnosis IV: accidental trauma
- Chapter 11 Differential diagnosis V: obstetric trauma
- Chapter 12 Differential diagnosis VI: normal variants
- Chapter 13 Evidence-based radiology and child abuse
- Chapter 14 Skeletal imaging strategies
- Chapter 15 Postmortem skeletal imaging
- Section II Abusive head and spinal trauma
- Section III Visceral trauma and miscellaneous abuse and neglect
- Section IV Diagnostic imaging of abuse in societal context
- Section V Technical considerations and dosimetry
- Index
- References
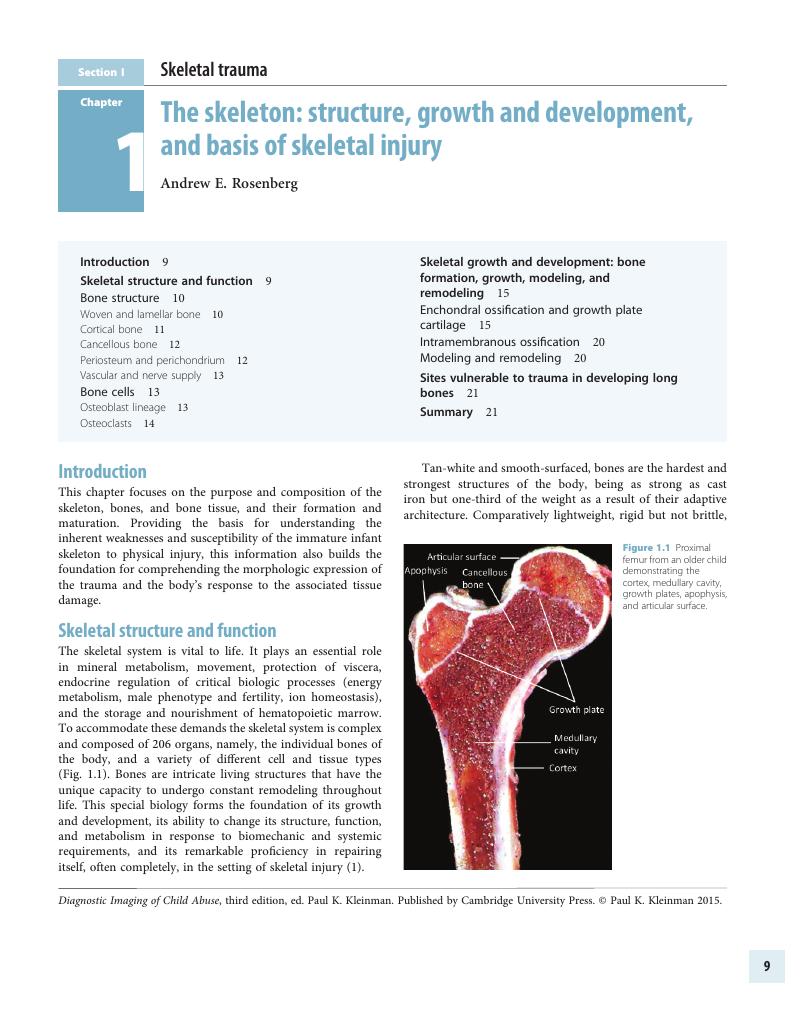
Summary
- Type
- Chapter
- Information
- Diagnostic Imaging of Child Abuse , pp. 9 - 22Publisher: Cambridge University PressPrint publication year: 2015
References
- 1
- Cited by



