Refine search
Actions for selected content:
106095 results in Materials Science
JMR volume 26 issue 6 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 6 / 28 March 2011
- Published online by Cambridge University Press:
- 25 March 2011, pp. b1-b4
- Print publication:
- 28 March 2011
-
- Article
-
- You have access
- Export citation
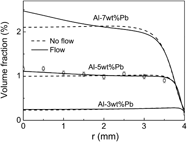
Convective effect on the solidification of hypermonotectic alloys
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 6 / 28 March 2011
- Published online by Cambridge University Press:
- 25 March 2011, pp. 832-836
- Print publication:
- 28 March 2011
-
- Article
- Export citation
JMR volume 26 issue 6 Cover and Front matter
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 6 / 28 March 2011
- Published online by Cambridge University Press:
- 25 March 2011, pp. f1-f5
- Print publication:
- 28 March 2011
-
- Article
-
- You have access
- Export citation
Blended elemental powder densification of Ti-6Al-4V by hot pressing
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 8 / 28 April 2011
- Published online by Cambridge University Press:
- 21 March 2011, pp. 965-969
- Print publication:
- 28 April 2011
-
- Article
- Export citation
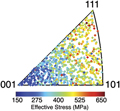
Correlation between crystallographic orientation and mechanical response in a three-dimensional β-Ti microstructure
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 8 / 28 April 2011
- Published online by Cambridge University Press:
- 21 March 2011, pp. 957-964
- Print publication:
- 28 April 2011
-
- Article
- Export citation
Tungsten alloying of the Ni(P) films and the reliability of Sn–3.5Ag/NiWP solder joints
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 7 / 14 April 2011
- Published online by Cambridge University Press:
- 21 March 2011, pp. 889-895
- Print publication:
- 14 April 2011
-
- Article
- Export citation
Solidification of nitrogen-atomized Al86Ni6Y4.5Co2La1.5 metallic glass
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 7 / 14 April 2011
- Published online by Cambridge University Press:
- 21 March 2011, pp. 944-950
- Print publication:
- 14 April 2011
-
- Article
- Export citation
Ti–Si–C–N thin films grown by reactive arc evaporation from Ti3SiC2 cathodes
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 7 / 14 April 2011
- Published online by Cambridge University Press:
- 16 March 2011, pp. 874-881
- Print publication:
- 14 April 2011
-
- Article
- Export citation
Silica-controlled structure and optical properties of zinc oxide sol–gel thin films
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 7 / 14 April 2011
- Published online by Cambridge University Press:
- 15 March 2011, pp. 882-888
- Print publication:
- 14 April 2011
-
- Article
- Export citation
Synthesis of TiO2–graphene composites via visible-light photocatalytic reduction of graphene oxide
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 8 / 28 April 2011
- Published online by Cambridge University Press:
- 15 March 2011, pp. 970-973
- Print publication:
- 28 April 2011
-
- Article
- Export citation
Cryomilling and spark plasma sintering of nanocrystalline magnesium-based alloy
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 7 / 14 April 2011
- Published online by Cambridge University Press:
- 15 March 2011, pp. 904-911
- Print publication:
- 14 April 2011
-
- Article
- Export citation
The effect of cooling rate on thermophysical properties of magnesium alloys
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 8 / 28 April 2011
- Published online by Cambridge University Press:
- 15 March 2011, pp. 974-982
- Print publication:
- 28 April 2011
-
- Article
- Export citation
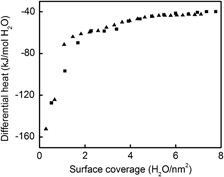
Surface enthalpy and enthalpy of water adsorption of nanocrystalline tin dioxide: Thermodynamic insight on the sensing activity
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 7 / 14 April 2011
- Published online by Cambridge University Press:
- 15 March 2011, pp. 848-853
- Print publication:
- 14 April 2011
-
- Article
- Export citation
JMR volume 26 issue 5 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 5 / 14 March 2011
- Published online by Cambridge University Press:
- 18 March 2011, pp. b1-b5
- Print publication:
- 14 March 2011
-
- Article
-
- You have access
- Export citation
JMR volume 26 issue 5 Cover and Front matter
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 5 / 14 March 2011
- Published online by Cambridge University Press:
- 18 March 2011, pp. f1-f4
- Print publication:
- 14 March 2011
-
- Article
-
- You have access
- Export citation
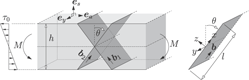
Continuum modeling of dislocation plasticity: Theory, numerical implementation, and validation by discrete dislocation simulations
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 5 / 14 March 2011
- Published online by Cambridge University Press:
- 18 March 2011, pp. 623-632
- Print publication:
- 14 March 2011
-
- Article
- Export citation
Structural properties of InN films grown in different conditions by metalorganic vapor phase epitaxy
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 6 / 28 March 2011
- Published online by Cambridge University Press:
- 11 March 2011, pp. 775-780
- Print publication:
- 28 March 2011
-
- Article
- Export citation
Effects of polypyrrole on the performance of nickel oxide anode materials for rechargeable lithium-ion batteries
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 7 / 14 April 2011
- Published online by Cambridge University Press:
- 11 March 2011, pp. 860-866
- Print publication:
- 14 April 2011
-
- Article
- Export citation
Growth-mode induced defects in epitaxial SrTiO3 thin films grown on single crystal LaAlO3 by a two-step PLD process
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 6 / 28 March 2011
- Published online by Cambridge University Press:
- 11 March 2011, pp. 770-774
- Print publication:
- 28 March 2011
-
- Article
- Export citation
Hybrid nanocomposite coatings for corrosion protection of low carbon steel: A substrate-integrated and scalable active–passive approach
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 6 / 28 March 2011
- Published online by Cambridge University Press:
- 11 March 2011, pp. 837-844
- Print publication:
- 28 March 2011
-
- Article
- Export citation