Summation
-
COVID-related first episode psychoses without SARS-CoV-2 infection are most probably stress-related and occurred only during the first wave of the pandemic.
-
First episode psychoses accompanying SARS-CoV-2 infection are of much more ambiguous origin. Inflammation seems to be important, yet in many patients only low-grade inflammation was found.
-
Short-term outcome in both groups of psychoses is very good. There are arguments for a favorable long-term prognosis, but until now data of follow up are lacking.
Consideration
-
Since this paper relies on case reports, the findings might be distorted by various aspects of reporting bias
-
Etiological considerations concerning these psychoses are hampered by the poor understanding of the pathophysiology of psychosis in general
-
More systematic investigations in the pathophysiology, epidemiology, and outcome of these psychoses are warranted
Introduction
The SARS-CoV-2 pandemic has led to an unprecedented medical, social and economic crisis. While COVID-19 was considered first to affect predominantly the respiratory tract, it soon became obvious that it is a multisystem illness (Dinakaran et al., Reference Dinakaran, Manjunatha, Naveen Kumar and Suresh2020; Steardo et al., Reference Steardo, Steardo and Verkhratsky2020; Yamamoto et al., Reference Yamamoto, Bolanos, Fiallos, Strand, Morris, Shahrokhinia, Cushing, Hopp, Tiwari, Hariri, Sokolov, Wheeler, Kaushik, Elsayegh, Eliashiv, Hedrick, Jafari, Johnson, Khorsandi, Gonzalez, Balakhani, Lahiri, Ghavidel, Amaya, Kloor, Hussain, Huang, Cormier, Wesson Ashford, Wang, Yaghobian, Khorrami, Shamloo, Moon, Shadi and Kateb2020). SARS-CoV-2 is considered to be ‘neurotropic’ as it can enter the central nervous system (CNS) and very early during the pandemic reports on neurologic and psychiatric manifestations started appearing (Mao et al., Reference Mao, Jin, Wang, Hu, Chen, He, Chang, Hong, Zhou, Wang, Miao, Li and Hu2020; Varatharaj et al., Reference Varatharaj, Thomas, Ellul, Davies, Pollak, Tenorio, Sultan, Easton, Breen, Zandi, Coles, Manji, Al-Shahi Salman, Menon, Nicholson, Benjamin, Carson, Smith, Turner, Solomon, Kneen, Pett, Galea, Thomas and Michael2020). The neuropsychiatric dimension of COVID-19 extended not only to people infected by the virus but as well to the general public. While the whole society suffered from the fears, restrictions and losses which came by the pandemic, those infected by the virus had to cope additionally with the effects of the virus on the body and the brain.
New-onset psychosis is one of the possible neuropsychiatric sequelae. Since the beginning of the pandemic, concerns were expressed about an increase of psychotic disorders (Troyer et al., Reference Troyer, Kohn and Hong2020; Watson et al., Reference Watson, Thomas, Solomon, Michael, Nicholson and Pollak2021) and the term ‘COVID-psychosis’ became popular, especially in the lay press. As early as January 2020, observations on occurrence of first-episode psychoses in conjunction with COVID-19 were made (Hu et al., Reference Hu, Su, Li, Zhou and Zhu2021) and since then numerous case reports have appeared. In this respect, there are two types of psychoses found in the literature. On the one hand, psychoses originated in otherwise healthy persons without being infected and on the other hand in those suffering from an acute infection with SARS-CoV-2. The first ones are not mere first-episode manifestations of schizophrenia in times of COVID-19, but in most instances distinct from schizophrenia due to symptoms, course, and outcome. These psychoses are mostly considered to be related to stress caused by the pandemic and are thematically intimately linked to issues of the pandemic. Of course, the question remains whether this demarcation from schizophrenia is really valid and sustainable in the long run.
There is no reason to assume that patients infected with SARS-CoV-2 do not suffer from stress and will not be prone for the same kind of psychosis. Yet in these patients, additional biological pathomechanisms must be considered. There are many arguments linking psychosis with infections and inflammation. Since the occurrence of schizophrenia-like psychoses during the pandemic of Spanish flu in 1918/19 (Menninger, Reference Menninger1919) especially viral infections are considered a possible origin of psychosis (Torrey & Peterson, Reference Torrey and Peterson1976; Yolken & Torrey, Reference Yolken and Torrey2008). Inflammation and autoimmunological processes might serve as decisive links between infection and development of psychotic symptoms (Kępińska et al., Reference Kępińska, Iyegbe, Vernon, Yolken, Murray and Pollak2020; Watson et al., Reference Watson, Thomas, Solomon, Michael, Nicholson and Pollak2021). In a substantial proportion of schizophrenic patients, elevated markers of inflammation have been found, and the ‘vulnerability-stress-inflammation’ model of schizophrenia was proposed (Müller et al., Reference Müller, Weidinger, Leitner and Schwarz2015; Müller, Reference Müller2018). The importance of immune processes is furthermore underlined by the Psychiatric Genomics Consortium finding of a variety of risk genes for schizophrenia regulating immune functions (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). Yet, up to now all these data connecting psychosis, viral infection and inflammation are just small pieces in the intriguing puzzle of the biological basis of psychosis which is still far from forming a coherent picture.
The combination of direct invasion into the brain, (hyper)inflammation and autoimmune processes is considered to be the cause for neurologic and neuropsychiatric sequelae for SARS-CoV-2 infection, too (Steardo et al., Reference Steardo, Steardo and Verkhratsky2020; Tancheva et al., Reference Tancheva, Petralia, Miteva, Dragomanova, Solak, Kalfin, Lazarova, Yarkov, Ciurleo, Cavalli, Bramanti and Nicoletti2020). Psychosis is especially linked to coronaviruses as already the former epidemics of coronaviruses SARS and MERS were suspected to increase psychotic disorders (Brown et al., Reference Brown, Gray, Lo Monaco, O’donoghue, Nelson, Thompson, Francey and Mcgorry2020). Beyond this, also the less pathogenic corona strains HKU1 and NL63 (causing mild flu-like disorders) have been linked to schizophrenia as antibodies against these were found more often in schizophrenic patients than in controls (Severance et al., Reference Severance, Dickerson, Viscidi, Bossis, Stallings, Origoni, Sullens and Yolken2011). For COVID-19, the situation is further complicated by an additional risk factor as some medications broadly used for treatment (especially corticosteroids and hydroxychloroquine) carry a risk for psychosis, too.
The aim of this observational study is to provide a comprehensive collection of reports of first-episode psychoses occurring during the pandemic, in persons with and without infection. A similar collection of cases with and without infection was performed by Watson et al. (Reference Watson, Thomas, Solomon, Michael, Nicholson and Pollak2021) early during the pandemic. While psychoses with infection were covered by some smaller reviews (Parra et al., Reference Parra, Juanes, Losada, Álvarez-Sesmero, Santana, Martí, Urricelqui and Rentero2020; Tariku & Hajure, Reference Tariku and Hajure2020) and a large systematic review (Smith et al., Reference Smith, Gilbert, Riordan, Helmke, Von Isenburg, Kincaid and Shirey2021), reviews for patients without infection lack until now. Thus, in this paper we want to fill this niche and review cases of first-episode psychoses during the pandemic in persons with as well as without COVID-19 infection. We will put special emphasis on the clinical presentation of the psychoses in the two groups of patients, its treatment and outcome, and the difficulties to diagnose these patients appropriately, a problem which applies especially for the patients with infection. We will present key clinical features in a descriptive comparison to illustrate similarities and differences.
Methods
We conducted our review following the SANRA-criteria for quality assessment of narrative review articles (Baethge et al., Reference Baethge, Goldbeck-Wood and Mertens2019). The literature search was conducted in PubMed, Embase and PsycINFO, using the search terms [(COVID-19 OR SARS-CoV-2 OR corona) AND (psychosis OR psychotic OR mania OR manic OR bipolar OR hallucination OR delusion or paranoi* OR cataton*)] searching for reports on first-episode psychotic disorders in the context of corona infection. The search covers publications until December 12th, 2021. Additionally, the bibliography of relevant papers was reviewed.
Inclusion and exclusion criteria
We selected all case reports or case series presenting these features: 1) first-episode psychosis; 2) other psychiatric disorders accompanied by prominent hallucinations or delusions (e.g. affective states with ‘psychotic symptoms’); 3) patients had to be either infected with SARS-CoV-2, proven by polymerase chain reaction (PCR) or antibody tests or patients whose first-episode psychosis was timely and thematically linked to the pandemic, but who tested negative for SARS-CoV-2; 4) patients with delirium were only included if there was a time span during which they were considered not to be delirious but psychotic. Excluded were 1) persons <18 years (for a review covering children and adolescents see Javed & Shad, Reference Javed and Shad2021); 2) patients with prior episodes presenting any psychotic symptoms; 3) patients with postpartum psychosis; 4) patients with ‘pure’ catatonia, without delusions or hallucinations (as e.g. published by (Amouri et al., Reference Amouri, Andrews, Heckers, Ely and Wilson2020; Mulder et al., Reference Mulder, Feresiadou, Fällmar, Frithiof, Virhammar, Rasmusson, Rostami, Kumlien and Cunningham2020)), due to the ambiguous aetiology of catatonia 5) cases of ‘pure’ mania without psychotic symptoms 6) cases with ‘pure’ delirium.
Extracted items
Personal data
Country of origin of the report, date of occurrence of psychosis, age, sex, previous psychiatric history, family history of psychosis.
Illness-related data
Psychopathology, COVID-19 as content of delusions, reason for admission to inpatient treatment (due to psychosis; due to COVID-19; due to both; due to other causes), acute onset (duration of untreated psychosis ≤ 7 days), presence of inflammation, C-reactive protein (CRP), cerebro-spinal fluid (CSF), CT or MRT scans (only changes with a potential relevance for COVID), duration of psychosis (≤1 week; >1 to ≤2 weeks; >2 to ≤4 weeks; 1 to ≤2 months; >2 months), severity of COVID-19 (no = no somatic symptoms; mild = outpatient treatment for COVID-19; severe = inpatient treatment except for treatment in intensive care unit (ICU) for COVID-19; very severe = treatment for COVID-19 in ICU), relation between diagnosis of infection/beginning of COVID-19 and onset of psychosis (patients with psychosis, but without somatic symptoms of COVID-19; psychosis before start of somatic symptoms; psychosis concomitant with somatic symptoms; psychosis when somatic symptoms had abated), diagnosis as provided by the authors ("psychosis (px)" was filled in if the authors provided no specific diagnosis or discussed differential diagnoses without making a decision).
Treatment and outcome
Duration of inpatient treatment, treatment with antipsychotics (AP) (dosage at discharge converted to risperidone equivalents based on the data of Leucht et al. (Reference Leucht, Samara, Heres, Patel, Woods and Davis2014)), treatment with drugs at risk for psychosis (chloroquine (CQ); hydroxychloroquine (HCQ); glucocorticoids (CS); treatment with other agents, if the authors considered it as putative causal), outcome (full remission; improvement; not improved), treatment response (fast response to treatment was documented if reported by the authors or remission occurred within 1 week).
Statistics
To characterise the two groups of patients, we performed descriptive statistics reporting mean, median and standard deviation for continuous variables and percentages for nominal variables. For comparing the two groups via hypothesis testing, we used chi-square tests with continuity correction for nominal and Mann–Whitney U-tests for continuous variables (Table 4).
Results
The search retrieved 4.035 hits (PubMed 762, Embase 1505, PsycINFO 1768). After exclusion of duplicates and irrelevant papers, 96 publications covering 146 patients could be found. Thirty-one papers comprising 62 patients dealt with patients developing psychosis without being infected with SARS-CoV-2, 67 papers covering 84 patients reported on patients with SARS-CoV-2 infection. Table 1 shows the distribution of the papers’ countries of origin. Cases were reported from all continents, with almost half (46.5%) of them originating from Europe and about a quarter (22.9%) from the USA.
Table 1. Geographic origin of 62 cases of psychosis without SARS-CoV-2 infection and 84 cases with infection

One paper presenting 10 cases (Parra et al., Reference Parra, Juanes, Losada, Álvarez-Sesmero, Santana, Martí, Urricelqui and Rentero2020) was included as far as the aggregate presentation of data allowed. There is one multicentre study (Valdés-Florido et al., Reference Valdés-Florido, López-Díaz, Palermo-Zeballos, Garrido-Torres, Álvarez-Gil, Martínez-Molina, Martín-Gil, Ruiz-Ruiz, Mota-Molina, Algarín-Moriana, Guzmán-Del Castillo, Ruiz-Arcos, Gómez-Coronado, Galiano-Rus, Rosa-Ruiz, Prados-Ojeda, Gutierrez-Rojas, Crespo-Facorro and Ruiz-Veguilla2021) which recruited systematically patients with brief psychotic disorders according to DSM-5 during the climax of the national lockdown in Spain (March 14th to May14th 2020), whose patients are not included in this paper for lack of comparability and data.
Patients without infection
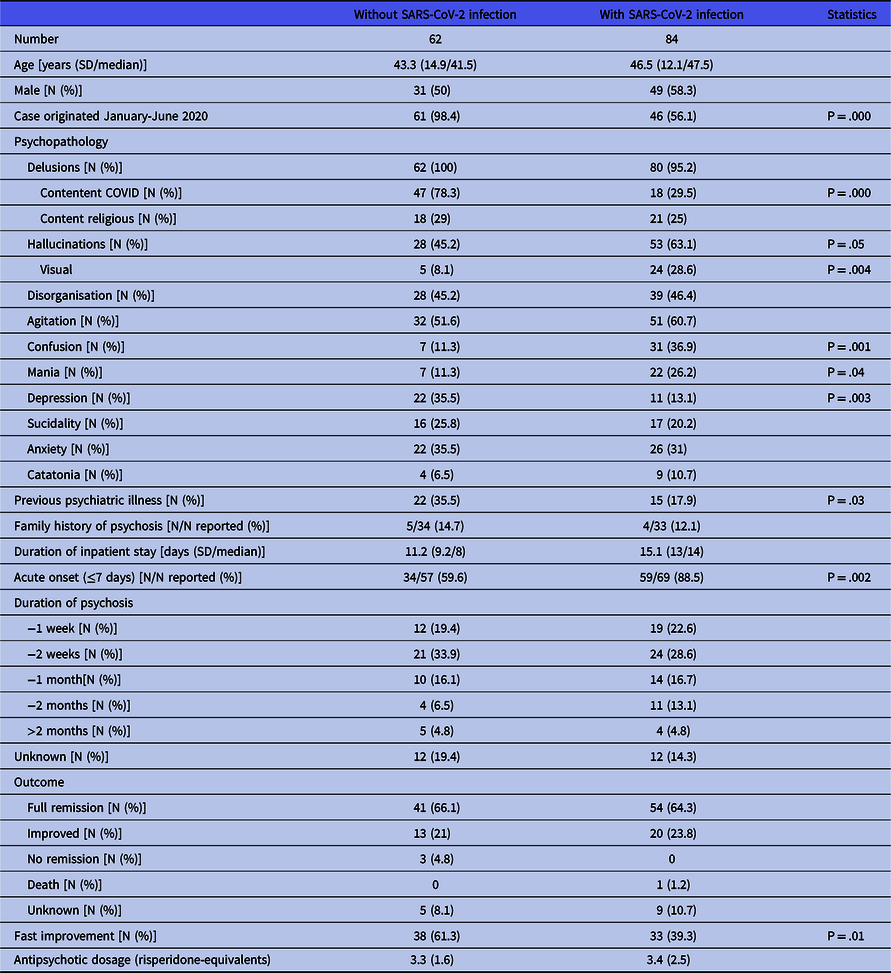
All cases of patients presenting first onset psychosis without being infected with SARS-CoV-2 originated from January to July 2020. Table 2 presents the individual data of the patients, Table 4 presents the aggregate findings. Patients without infection were 43.3 years old as a mean (41.5 as median) and equally distributed concerning sex. Psychopathology was characterised by delusions which were typically centred on COVID-related themes in more than three-quarters (78.3%) of patients. Common issues were the conviction of being infected (despite negative test results), the fear to spread the virus and such being responsible for the death of others or the urge to fulfil divine commands to save the world from the virus. Religious contents were found in 29% of patients. Hallucinations (mostly acoustic), disorganisation, and agitation were present in about 40 to 50% of patients, anxiety and depression in about a third of patients. Twenty (35.7%) patients had a history of previous psychiatric illness, half of them anxiety disorders.
Table 2. Reports on patients with COVID-19-related psychosis without SARS-CoV-2 infection

Column headings: Country: Code according to ISO 3166 Alpha-3; Inpat. (d): inpatient treatment (days); Duration px: duration of psychosis (1: ≤1 week; 2: >1–2 weeks; 3: >2-4 weeks; 4: 1–2months; 5: >2months); Outcome (1: full remission, 2: improvement, 3: no improvement); Treatment AP (treatment with antipsychotics at discharge, risperidone equivalents); Fast response (to treatment: as stated by the authors or within ≤ 1 week); Psych. hist.: history of previous psychiatric illness according to categories of ICD-10 (diagnosis is set in italics); COVID-related delusions are described if present.
Abbreviations: ATPD: acute and transient psychotic disorder; BPD: brief psychotic disorder; BRP: brief reactive psychosis; CP: cycloid psychosis; Del.: delusion; ECT: electroconvulsive therapy; F: female; F1 mental and behavioural disorders due to psychoactive substance use; F3 mood [affective] disorders, F4 neurotic, stress-related and somatoform disorders, F7 mental retardation; F8 disorders of psychological development; HCQ: hydroxychloroquine; HCW: health care worker; M: male; MDE: major depressive episode; NA: data not available; Outp: treatment as outpatient; Px: psychosis; sy: symptoms
Most patients were admitted to hospital for psychosis and treated as inpatients, only 6 (9.7%) (Martin, Reference Martin2020; Mehra et al., Reference Mehra, Rani, Sahoo, Parveen, Singh, Chakrabarti and Grover2020; Oloniniyi et al., Reference Oloniniyi, Ibigbami, Amiola, Esan and Esan2021) were treated as outpatients. Inpatient treatment ranged between 1 and 42 days, with a mean of 11.2 (median 8) days. Duration of psychosis was ≤2 weeks in more than 70% of patients. Most patients were treated with antipsychotics (AP), the mean dose equivalent was 3.3 mg risperidone. For four patients, no AP were used (Martin, Reference Martin2020; Ambar Akkaoui et al., Reference Ambar Akkaoui, Lejoyeux and Geoffroy2021). Six patients discontinued the medication with APs within 1 month (D’Agostino et al., Reference D’Agostino, D’angelo, Giordano, Cigognini, Chirico, Redaelli and Gambini2020; Loehde & Novakovic, Reference Loehde and Novakovic2021; Oloniniyi et al., Reference Oloniniyi, Ibigbami, Amiola, Esan and Esan2021; Shakya & Upadhaya, Reference Shakya and Upadhaya2021), of whom one suffered a relapse after 1 month (Loehde & Novakovic, Reference Loehde and Novakovic2021).
Outcome with full remission occurred in two-thirds of patients, and almost as many reports describe a fast response to antipsychotic treatment. Only the paper of Deshpande & Livingstone (Reference Deshpande and Livingstone2021) presents three patients with slow response to treatment and long inpatient stays. These patients were selected for reporting among others because of their advanced age and to illustrate the deleterious effects of social isolation on elder persons normally living an active life. There are more reports of this kind (de Oliveira, Reference De Oliveira2021; Lynch & Bastiampillai, Reference Lynch and Bastiampillai2021). There is no information about the further fate of patients except for two, who were reported to be well after about half a year (de Oliveira, Reference De Oliveira2021; Marouda et al., Reference Marouda, Mantonakis and Kollias2021).
Two-thirds of patients were diagnosed with either ‘brief psychotic disorder’ (BPD) according to DSM-5 or ‘acute and transient psychotic disorder’ (ATPD) according to ICD-10. This includes five patients diagnosed with ‘brief reactive psychosis’ and five patients with ‘cycloid psychosis’, a type of brief psychosis most closely related to ICD-10 ATPD, polymorphic subtype (Giné Servén et al., Reference Giné Servén, Martinez Ramirez, Boix Quintana, Petrizan Aleman, Barón Fernández, Fernández Corcuera, Serra Buil and Cañete Crespillo2021). Some patients presented features complicating diagnosis: On two occasions (Dhir et al., Reference Dhir, Khalid, Salcedo and Shanbour2020; Ambar Akkaoui et al., Reference Ambar Akkaoui, Lejoyeux and Geoffroy2021), self-treatment with different drugs might have contributed to psychosis. Cannabis consumption with low intensity was reported in one case (Marouda et al., Reference Marouda, Mantonakis and Kollias2021), with high intensity in two cases (Doufik et al., Reference Doufik, Ouhmou, Bouraoua, Laaraj, Mouhadi and Rammouz2021; Marín et al., Reference Marín, Pérez De Mendiola, Fernández and Chart2021). The authors of the latter paper attributed psychotic symptoms to cannabis withdrawal syndrome (due to lockdown and quarantine). Two patients received a schizophrenic spectrum diagnosis. Belvederi Murri et al. (Reference Belvederi Murri, Zotos, Cantarelli, Berardi, Curtarello, Folesani, Gullotta, Bertolini, Girotto, Carozza and Grassi2021) reported the case of a female student of Chinese origin living in Italy who became acutely psychotic during the first lockdown and in whom discreet psychotic symptoms had been present already 10 months before. The other patient was a male with a 1-month history of changed behaviour who responded quickly on treatment with AP (Doufik et al., Reference Doufik, Ouhmou, Bouraoua, Laaraj, Mouhadi and Rammouz2021). Eight times (12.8%) the diagnosis ‘psychosis’ was given without further specification.
Patients with infection
More than half of the cases (56.1%) dated from the beginning of the pandemic (January to June 2020), but there is an ongoing stream of publications. Table 3 presents the individual data of patients, Table 4 the aggregate findings and Table 5 the COVID-related data. Patients with infection were 46.5 years old as a mean (median 47.5 years). Males prevailed with 58.3%. The most prominent symptoms of psychosis were delusions (95.2%), agitation (60.7%), disorganisation (46.4%) and confusion (36.9%). Delusions thematically centred on the pandemic were found in 29.5%, with religious content in 25%. Almost two-thirds suffered from hallucinations, of whom 33 (39.2%) had acoustic hallucinations, 9 (10.7%) visual ones and 15 (17.9) both types. Ten patients (11.9%) had a history of previous psychiatric disorders, most commonly substance use disorders (six patients), of whom two had an active use of cannabis (Jaworowski et al., Reference Jaworowski, Weiser, Gropp and Malka2020; Panariello et al., Reference Panariello, Bassetti, Radice, Rossotti, Puoti, Corradin, Moreno and Percudani2020a) and one was substituted with methadone (Ferrando et al., Reference Ferrando, Klepacz, Lynch, Tavakkoli, Dornbush, Baharani, Smolin and Bartell2020).
Table 3. Reports on patients with SARS-CoV-2 infection

Column headings: Country: Code according to ISO 3166 Alpha-3; COVID severity: 1: no somatic symptoms; 2: outpatient treatment for COVID-19; 3: inpatient treatment except for treatment in intensive care unit (ICU) for COVID-19; 4: treatment for COVID-19 in ICU; Somatic sy/px: 1: patients with psychosis, but without somatic symptoms of COVID-19; 2: psychosis before start of somatic symptoms; 3: psychosis concomitant with somatic symptoms; 4: psychosis when somatic symptoms had abated; Pat. (d) : inpatient treatment (days); Duration px: duration of psychosis (1: ≤1 week; 2: >1-2 weeks; 3: >2-4 weeks ; 4: 1-2 months; 5: >2months); Outcome (1: full remission, 2: improvement, 3: no improvement); Treatment AP (treatment with antipsychotics at discharge, risperidone equivalents); Fast response (to treatment: as stated by the authors or ≤1 week); Psych. hist.: history of previous psychiatric illness according to categories of ICD-10 (diagnosis is set in italics).
Abbreviations: AP: antipsychotics; ATPD: acute and transient psychotic disorder; BPD: brief psychotic disorder; BRP: brief reactive psychosis; CLOCC: cytotoxic lesion of corpus callosum; CP: cycloid psychosis; CS: corticosteroids; CQ: chloroquine; ECT: electroconvulsive therapy; F: female; F1 mental and behavioural disorders due to psychoactive substance use; F3 mood [affective] disorders, F4 neurotic, stress-related and somatoform disorders, F7 mental retardation; F8 disorders of psychological development; HCQ: hydroxychloroquine; HCW: health care worker; M: male; MDE: major depressive episode; NA: data not available; Outp: treatment as outpatient; pat.: patient; Px: psychosis; sy: symptoms;.
Table 4. Patients with and without SARS-CoV-2 infection: demographic and illness-related variables, psychopathology, treatment, and outcome. Statistic comparison between groups (chi-square tests with continuity correction, Mann–Whitney U-test)
Abbreviations: SD: standard deviation.
Table 5. Patients with SARS-CoV-2 infection: illness, investigations, and treatment variables

Abbreviations: SD; standard deviation; px: psychosis.
The majority of patients (60.7%) came to hospital because of psychosis, while a quarter was admitted for COVID symptoms. Only four patients were treated for psychosis as outpatients. Most patients received APs. Patients not treated with APs suffered either from a defined organic brain disorder (Panariello et al., Reference Panariello, Bassetti, Radice, Rossotti, Puoti, Corradin, Moreno and Percudani2020a; Alvarez-Cisneros et al., Reference Alvarez-Cisneros, Lara-Reyes and Sansón-Tinoco2021; Elfil et al., Reference Elfil, Selby, Van Schooneveld and Fadul2021; McAlpine et al., Reference Mcalpine, Lifland, Check, Angarita, Ngo, Pleasure, Wilson, Spudich, Farhadian and Bartley2021), from catatonia (Caan et al., Reference Caan, Lim and Howard2020; Zain et al., Reference Zain, Muthukanagaraj and Rahman2021) or were thought to have a psychosis due to an offending drug, which was then withdrawn. Three patients were treated with electroconvulsive therapy (ECT) (Chacko et al., Reference Chacko, Job, Caston, George, Yacoub and Cáceda2020; Vepa et al., Reference Vepa, Saleem, Dharmaraj and Afzaal2020; Austgen et al., Reference Austgen, Meyers, Gordon and Livingston2021). The mean dosage of antipsychotics at discharge was 3.4 mg risperidone equivalents. Sixteen patients (19%) did not receive antipsychotics at discharge or stopped it within the next month. Duration of psychosis was ≤2 weeks in 51.2% of patients, only in four (4.8%) the duration exceeded 2 months. Inpatient treatment ranged between 2 and 67 days with a mean of 15.1 days (median 14).
Outcome was full remission in 64.3%, and the rest was rated as improved. A fast improvement was observed in 33 (39.3%) patients. One patient showed a foudroyant course of COVID-19 and died (Elkhaled et al., Reference Elkhaled, Ben Abid, Akhtar, Abukamar and Ibrahim2020), another patient’s psychosis remitted, but she died some days later for unknown reasons (Borovina et al., Reference Borovina, Mastelić, Glavina and Glavina2021).
In more than half of the patients, the somatic symptoms of COVID were either absent (16.7%) or that mild that no hospital treatment would have been necessary. Eight (9.5%) required treatment in an intensive care unit (ICU). Psychosis started in 35.7% of patients while somatic symptoms were present and in 32.1% after somatic symptoms had abated. On 2 occasions, psychosis manifested before somatic symptoms (Tuna et al., Reference Tuna, Salman and Darcin2020; Santos et al., Reference Santos, Alho, Costa, Ferreira and Sêco2021). The time range from start of somatic symptoms to start of psychosis was 0 to 60 days, with a mean of 13.4 (median 14). The aggregate paper of Parra et al. (Reference Parra, Juanes, Losada, Álvarez-Sesmero, Santana, Martí, Urricelqui and Rentero2020) reported a mean time span of ‘bigger than 14 days’.
The diagnoses varied considerably (see Table 3). Most often, a diagnosis of psychosis without further specification was made or differential diagnoses were discussed without a decision (N = 32, 38.1%). In 14 (16.7%) patients, ATPD/BPD were diagnosed, among these four cases of brief reactive psychosis (Jaworowski et al., Reference Jaworowski, Weiser, Gropp and Malka2020; Mollà Roig, Reference Mollà Roig2021). Mania with psychotic features was diagnosed in 10 patients (11.9%) and depression with psychotic features in one patient. Psychosis due to a general medical condition (PDGMC) was chosen in 12 (14.3%) patients, of whom 6 presented with a defined organic lesion of the brain: two cases with cytotoxic lesions of the corpus callosum (CLOCC), of whom one ended fatally (Elkhaled et al., Reference Elkhaled, Ben Abid, Akhtar, Abukamar and Ibrahim2020), the other remitted fully within a few days (Sen et al., Reference Sen, Yesilkaya and Balcioglu2021), further three cases with suspected meningoencephalitis (Bernard-Valnet et al., Reference Bernard-Valnet, Pizzarotti, Anichini, Demars, Russo, Schmidhauser, Cerutti-Sola, Rossetti and Du Pasquier2020; Ariza-Varón et al., Reference Ariza-Varón, Beltrán, Marín-Medina, González and Ávila2021; McAlpine et al., Reference Mcalpine, Lifland, Check, Angarita, Ngo, Pleasure, Wilson, Spudich, Farhadian and Bartley2021), one case of encephalopathy (Jozuka et al., Reference Jozuka, Kimura, Uematsu, Fujigaki, Yamamoto, Kobayashi, Kawabata, Koike, Inada, Saito, Katsuno and Ozaki2021) and one case with NMDA autoantibody encephalitis (Panariello et al., Reference Panariello, Cellini, Speciani, De Ronchi and Atti2020b). Substance-induced psychotic disorder was diagnosed in six (8.3%) cases, CS (Grover et al., Reference Grover, Sahoo, Rijal and Mehra2021; Kazi & Hoque, Reference Kazi and Hoque2021), CQ (Benjelloun et al., Reference Benjelloun, Otheman and El Kettani2020), HCQ (Boulos et al., Reference Boulos, Newman and Newman2020), favipiravir (Duyan & Ozturan, Reference Duyan and Ozturan2021) and cannabis (Kaggwa et al., Reference Kaggwa, Bongomin, Najjuka, Rukundo and Ashaba2021) being considered as offending substances. Beyond these cases, some more authors assumed strong evidence for psychosis being substance induced, without expressing it through diagnosis. This concerned CS (Desai et al., Reference Desai, Sheikh and Belzie2021; Jiménez-Fernández et al., Reference Jiménez-Fernández, Solis, Martínez-Reyes, Alvarado-Dafonte, Soldado-Rodríguez and Rodríguez-Natal2021; Russo et al., Reference Russo, Consoli, De Rosa, Calisi, Dono, Carrarini, Onofrj, De Angelis and Sensi2021), CS + HCQ (Correa-Palacio et al., Reference Correa-Palacio, Hernandez-Huerta, Gómez-Arnau, Loeck and Caballero2020) and azithromycin, which was thought to be responsible for catatonia (Caan et al., Reference Caan, Lim and Howard2020). Only twice the diagnosis of a schizophreniform disorder was made, in one patient with a prodromal phase 1 month before COVID-19 (Tuna et al., Reference Tuna, Salman and Darcin2020), the other for behavioural changes after infection with psychotic symptoms occurring 2 months later (DeLisi, Reference Delisi2021).
Analysis of CSF was performed in 25 patients, with pathologic results in 6 patients (Bernard-Valnet et al., Reference Bernard-Valnet, Pizzarotti, Anichini, Demars, Russo, Schmidhauser, Cerutti-Sola, Rossetti and Du Pasquier2020; Noone et al., Reference Noone, Cabassa, Gardner, Schwartz, Alpert and Gabbay2020; Panariello et al., Reference Panariello, Bassetti, Radice, Rossotti, Puoti, Corradin, Moreno and Percudani2020a; Parra et al., Reference Parra, Juanes, Losada, Álvarez-Sesmero, Santana, Martí, Urricelqui and Rentero2020; Austgen et al., Reference Austgen, Meyers, Gordon and Livingston2021; McAlpine et al., Reference Mcalpine, Lifland, Check, Angarita, Ngo, Pleasure, Wilson, Spudich, Farhadian and Bartley2021). Detection of SARS-CoV-2 in the brain was never reported; in one patient (Noone et al., Reference Noone, Cabassa, Gardner, Schwartz, Alpert and Gabbay2020), antibodies against the virus were found in the CSF. Neuroimaging with CT or MRI was performed in 65 patients with pathologic results in 5 (7.7%). Presence of delirium as a clinical sign of compromised brain function was a frequent issue, even when cases of mere delirium were excluded. Yet there were patients who showed transition from delirium to psychosis (Caan et al., Reference Caan, Lim and Howard2020; Clouden, Reference Clouden2020; Lim et al., Reference Lim, Janaway, Costello, Trip and Price2020; Majadas et al., Reference Majadas, Pérez, Casado-Espada, Zambrana, Bullón and Roncero2020; Khatib et al., Reference Khatib, Mahgoub, Elzain, Ahmed, Mohamed and Nashwan2021) and vice versa (Elkhaled et al., Reference Elkhaled, Ben Abid, Akhtar, Abukamar and Ibrahim2020; Panariello et al., Reference Panariello, Bassetti, Radice, Rossotti, Puoti, Corradin, Moreno and Percudani2020a; Jozuka et al., Reference Jozuka, Kimura, Uematsu, Fujigaki, Yamamoto, Kobayashi, Kawabata, Koike, Inada, Saito, Katsuno and Ozaki2021) or states alternating between delirium and psychosis (Bernard-Valnet et al., Reference Bernard-Valnet, Pizzarotti, Anichini, Demars, Russo, Schmidhauser, Cerutti-Sola, Rossetti and Du Pasquier2020). Sometimes, delirium was reported as concomitant disorder or as a differential diagnosis to psychosis (Bernard-Valnet et al., Reference Bernard-Valnet, Pizzarotti, Anichini, Demars, Russo, Schmidhauser, Cerutti-Sola, Rossetti and Du Pasquier2020; Gillett & Jordan, Reference Gillett and Jordan2020; Losee & Hanson, Reference Losee and Hanson2020; Haddad et al., Reference Haddad, Alabdulla, Latoo and Iqbal2021; Parker et al., Reference Parker, Slan, Shalev and Critchfield2021; Reinfeld & Yacoub, Reference Reinfeld and Yacoub2021), and in more than a third of all patients, confusion was documented.
Blood tests were performed in almost every patient, yet not all reports were concise regarding the presence of inflammatory markers. Signs of inflammation were found in 47 (78.3%) of 60 evaluable patients. The most frequently reported parameter of inflammation was CRP, being above normal in 34/58 (58.6%) patients.
The possible effects of perceived stress were discussed in 27/55 (49.1%) of patients, yet only 7 times they were thought to be decisive (Haddad et al., Reference Haddad, Al Abdulla, Latoo and Iqbal2020; Jaworowski et al., Reference Jaworowski, Weiser, Gropp and Malka2020; Parra et al., Reference Parra, Juanes, Losada, Álvarez-Sesmero, Santana, Martí, Urricelqui and Rentero2020; Baral et al., Reference Baral, Adhikari, Karki, Champine and Sud2021; Mollà Roig, Reference Mollà Roig2021). On the other hand, there were also reports which underlined the absence of any concern (Ferrando et al., Reference Ferrando, Klepacz, Lynch, Tavakkoli, Dornbush, Baharani, Smolin and Bartell2020; Majadas et al., Reference Majadas, Pérez, Casado-Espada, Zambrana, Bullón and Roncero2020; Alba et al., Reference Alba, Coll, Sáez, Alonso, Pérez, Palma, Vallés and Ortiz2021; Parker et al., Reference Parker, Slan, Shalev and Critchfield2021).
Comparison of patients with and without SARS-CoV-2 infection (Table 4) revealed some significant differences regarding psychopathology. Patients with infection showed less often delusional contents thematically linked to COVID (29.5 vs 78.3%, p = .000) and less often depressive symptoms (13.1 vs 35.5%, p = .003), but more frequently hallucinations (63.1 vs 45.2%, p = .05), especially visual ones (28.6 vs 8.1%, p = .004), confusion (36.9 vs 11.3%, p = .001) and mania (26.2 vs 11.3%, p = .04). They had less often a personal history of other psychiatric illnesses (17.9 vs 35.5%, p = .03) and showed less often a fast improvement of psychosis (39.3 vs 61.3%, p = .01).
Discussion
Our literature search revealed 146 cases of first-episode psychoses, 62 without and 84 with SARS-CoV-2 infection, coming from all parts of the world. Though this certainly represents only a tiny segment of occurring morbidity, it might serve to illustrate the characteristics of psychoses associated with COVID-19.
All patients without infection presented delusions and about half of them hallucinations and disorganisation. Delusions were thematically linked to COVID-19, with conviction to be infected or delusional fears of impending catastrophe, triggered by isolation, quarantine and economic problems. Especially in the first months of the pandemic, health care workers (HCW) were reported to be at heightened risk for physical and mental disorders (Salazar de Pablo et al., Reference Salazar De Pablo, Vaquerizo-Serrano, Catalan, Arango, Moreno, Ferre, Shin, Sullivan, Brondino, Solmi and Fusar-Poli2020). Six (9.7%) of these patients belonged to this profession.
Course of illness was mostly favourable. More than 50% had a duration of psychosis of ≤2 weeks; full remission and rapid response to treatment was reported in more than 60% of cases. No improvement was only found in three patients: one chronic abuser of cannabis who left the hospital against advice after 9 days and the two patients of Deshpande & Livingstone (Reference Deshpande and Livingstone2021) already described. Most patients were treated with APs, some received only tranquilisers. Patients mostly were diagnosed as acute transient psychotic disorders (ATPD; F23 according to ICD-10) or brief psychotic disorder (BPD according to DSM-5).
The diagnosis ATPD/BPD is rare with an incidence of 4–6.7 cases per 100.000 persons/year (Castagnini & Foldager, Reference Castagnini and Foldager2013; Queirazza et al., Reference Queirazza, Semple and Lawrie2014; Fusar-Poli et al., Reference Fusar-Poli, Cappucciati, Bonoldi, Hui, Rutigliano, Stahl, Borgwardt, Politi, Mishara, Lawrie, Carpenter and Mcguire2016) and so is research on this diagnosis. ATPD comprises 6 variations of brief psychotic disorders and allows a duration of up to 3 months, whereas psychosis in BPD must not be present longer than 1 month and provides three specifiers (with or without marked stressors, with postpartum onset). Though the diagnostic criteria of these two disorders differ in duration and specification of symptoms, the sociographic characteristics and outcome of the patients were found to be quite similar (Pillmann et al., Reference Pillmann, Haring, Balzuweit, Blöink and Marneros2002; Fusar-Poli et al., Reference Fusar-Poli, Cappucciati, Bonoldi, Hui, Rutigliano, Stahl, Borgwardt, Politi, Mishara, Lawrie, Carpenter and Mcguire2016). Prognosis of ATPD/BPD generally is considered to be much better than in schizophrenia (Marneros et al., Reference Marneros, Pillmann, Haring, Baizuweit and Blöink2003), yet studies vary considerably concerning the proportion of patients who relapse. A meta-analytic study calculated the mean risk for relapse with 54% for ATPD and 53% for BPD over a period of 3 years (Castagnini & Fusar-Poli, Reference Castagnini and Fusar-Poli2017) and conversion to schizophrenia (median follow-up time 24 months) occurred in ATPD in 19% and in BPD in 15% (Fusar-Poli et al., Reference Fusar-Poli, Cappucciati, Bonoldi, Hui, Rutigliano, Stahl, Borgwardt, Politi, Mishara, Lawrie, Carpenter and Mcguire2016).
Regarding age, our sample differed from other samples of ATPD/BPD in the literature, as in these (Marneros et al., Reference Marneros, Pillmann, Haring, Baizuweit and Blöink2003; Castagnini & Foldager, Reference Castagnini and Foldager2013; Queirazza et al., Reference Queirazza, Semple and Lawrie2014), mean age of onset was reported to be 35–37 years, whereas our patients aged 43.3 years as a mean. The proportion of females in our sample (50%) lies on the lower side of the range reported by these studies spanning from 45.5% (Queirazza et al., Reference Queirazza, Semple and Lawrie2014) to 50% (Castagnini & Foldager, Reference Castagnini and Foldager2013) to 79% (Marneros et al., Reference Marneros, Pillmann, Haring, Baizuweit and Blöink2003).
Factors heralding good prognosis of ATPD/BPD are female sex, higher age, a sudden onset of symptoms, short duration of symptoms and presence of stressful triggers (Pillmann et al., Reference Pillmann, Haring, Balzuweit, Blöink and Marneros2002; Marneros et al., Reference Marneros, Pillmann, Haring, Baizuweit and Blöink2003; Queirazza et al., Reference Queirazza, Semple and Lawrie2014; Rusaka & Rancāns, Reference Rusaka and Rancāns2014; Stephen & Lui, Reference Stephen and Lui2021). The importance of stressful events preceding the psychosis has lost momentum since the diagnosis of brief reactive psychosis was discarded with the change from ICD-9 to ICD-10 and DSM-III-R to DSM-IV (Ungvari et al., Reference Ungvari, Leung and Tang2000). In actual schemes, presence of stressors can be coded only as a specifier. This might be justified as some case series found only a minority of patients in which preceding stressors could be found: 43% in a sample of Marneros et al. (Reference Marneros, Pillmann, Haring, Baizuweit and Blöink2003) or only 12% in a sample of Pillmann et al. (Reference Pillmann, Haring, Balzuweit, Blöink and Marneros2002). In contrast, stress seems of outmost importance in our sample and is documented in all cases. This is also reflected by more than three-quarters of patients integrating COVID themes in delusional thinking. This fulfils a central criterion Jaspers proposed for reactive psychoses, that the content of the psychosis reflects the precipitating event (Jaspers, Reference Jaspers1913; Ungvari & Mullen, Reference Ungvari and Mullen2000). Considering this, the cases here display a very ‘reactive’ sample of ATPD/BPD. In this vein fits the unexpected finding that all reports originated between January and July 2020, representing the occurrence of the first wave of COVID-19 in most countries. While case reports on psychotic patients infected with SARS-CoV-2 peaked in the first half-year of the pandemic but continued to appear until now, no single report of psychosis without infection was to be found after July 2020. It seems that only the threat and awe of the first wave of COVID-19 – a situation that in most parts of the world nobody has encountered before in his lifetime – was able to elicit these psychotic reactions. As people became accustomed to this threat, this type of psychosis apparently vanished, at least in the literature.
Taken all together, the good outcome of our patients in their index episode, the generally good prognosis of a diagnosis of ATPD/BPD, higher age, short duration of psychosis and the pathogenetic importance of extreme stress, a good outcome for these cases should be assumed in the long run too. Yet we should keep in mind that ‘good’ outcome of ATPD/BPD means ‘better than in schizophrenia’ and that the risk of relapse for ATPD/BPD was calculated to be about 50% within 3 years (Castagnini & Fusar-Poli, Reference Castagnini and Fusar-Poli2017) (albeit probably in samples with less ‘reactivity’). Compared to patients with infection, these patients displayed more often a history of another psychiatric illness, especially anxiety disorders. This could hint to a greater psychosocial vulnerability of this group. In both groups, a family history of psychotic disorders was rare (with a low grade of reporting). Until now, no publication concerning follow-up information for these patients could be spotted.
Concerning psychosis among SARS-CoV-2 infected patients, we found 67 papers covering 84 patients. Comparing with the systematic review by Smith et al. (Reference Smith, Gilbert, Riordan, Helmke, Von Isenburg, Kincaid and Shirey2021), which presents a somewhat smaller sample (N = 48) and is more restrictive concerning exclusion of cases overlapping with delirium, data on demographics and psychopathology are quite similar. Patients were somewhat older (46.5 years) than patients without infection, with a slight preponderance of males (58.3%). Most of them showed delusions, but a content concerning the pandemic was reported far less often as in the patients without infection. On the other hand, rates of confusion and hallucinations were higher, especially of visual hallucinations. This is an argument for an organic pathogenesis, as visual hallucinations are found there more frequently (Feinstein & Ron, Reference Feinstein and Ron1998; American Psychiatric Association, 2013). Also, in delirium visual hallucinations are more prevalent than auditory ones (Webster & Holroyd, Reference Webster and Holroyd2000) and the higher prevalence of confusion signals an overlap with delirium as well. Many authors reported problems delimiting delirium from psychosis. Delirium is the most prominent psychiatric symptom among hospitalised patients with COVID-19 (Manca et al., Reference Manca, De Marco and Venneri2020; Mukaetova-Ladinska & Kronenberg, Reference Mukaetova-Ladinska and Kronenberg2020; Rogers et al., Reference Rogers, Chesney, Oliver, Pollak, Mcguire, Fusar-Poli, Zandi, Lewis and David2020). The overlap of delirium with psychosis in the course of viral infections is not uncommon. Menninger reporting on the influenza pandemic of 1918/19 (Menninger, Reference Menninger1919) already found cases where delirium and psychosis were indistinguishable. Smith et al. (Reference Smith, Gilbert, Riordan, Helmke, Von Isenburg, Kincaid and Shirey2021) also stated in their review that separation of delirium from psychosis is poorly accomplished and warrants further methodological efforts. As already shown, some cases changed between delirium and psychosis. Symptoms of delirium seem to be distributed in a continuous rather than a categorial mode. While the occurrence of delirium (and also of neurologic symptoms) shows a clear correlation with severity of somatic symptoms of COVID-19 (Mao et al., Reference Mao, Jin, Wang, Hu, Chen, He, Chang, Hong, Zhou, Wang, Miao, Li and Hu2020; Mukaetova-Ladinska & Kronenberg, Reference Mukaetova-Ladinska and Kronenberg2020; Tancheva et al., Reference Tancheva, Petralia, Miteva, Dragomanova, Solak, Kalfin, Lazarova, Yarkov, Ciurleo, Cavalli, Bramanti and Nicoletti2020; Frontera et al., Reference Frontera, Sabadia, Lalchan, Fang, Flusty, Millar-Vernetti, Snyder, Berger, Yang, Granger, Morgan, Patel, Gutman, Melmed, Agarwal, Bokhari, Andino, Valdes, Omari, Kvernland, Lillemoe, Chou, Mcnett, Helbok, Mainali, Fink, Robertson, Schober, Suarez, Ziai, Menon, Friedman, Friedman, Holmes, Huang, Thawani, Howard, Abou-Fayssal, Krieger, Lewis, Lord, Zhou, Kahn, Czeisler, Torres, Yaghi, Ishida, Scher, De Havenon, Placantonakis, Liu, Wisniewski, Troxel, Balcer and Galetta2021), no such correlation exists for psychosis, which occurred even in infections without clinical symptoms, yet in these significantly less often accompanied by confusion (7.1% vs 44.1% in the rest, p = .02, post hoc analysis).
Psychosis developing during presence of somatic symptoms of COVID-19 was the most common pattern, prevailing in more than a third of patients, followed by onset of psychosis after abating of somatic symptoms. The mean time lag between onset of somatic symptoms and onset of psychosis was 13.4 days (median 10) with a range from 0 to 60 days. This equates the mean time reported for neurologic complications of COVID-19, which is 12.8 days (range 0–56) (Podury et al., Reference Podury, Srivastava, Khan, Kakara, Tandon, Shrestha, Freeland, Wen and Sriwastava2021), suggesting that the similar mechanisms might be at work. Two patients showed an appearance of psychosis prior to somatic symptoms. Psychosis manifested 2 days (Santos et al., Reference Santos, Alho, Costa, Ferreira and Sêco2021) and a month before somatic symptoms (Tuna et al., Reference Tuna, Salman and Darcin2020). These patients deserve attention because they highlight the possibility that psychosis can be the first manifestation of SARS-CoV-2 infection. They also illustrate the general difficulties to single out the timely relation between somatic symptoms of COVID and beginning of psychosis, either because patients do not have symptoms or do not complain about it, or authors do not report about it. The case described by Tuna et al. (Reference Tuna, Salman and Darcin2020) is potentially misplaced in this review but was included because it also highlights the diagnostic ambiguities accompanying these cases. A 52-year-old woman was admitted for trying to jump out of a balcony under the influence of command hallucinations. After admission, she was found out to have pneumonia and the PCR test was positive for COVID-19. Yet later, the patient reported that psychotic symptoms already started a month earlier. Therefore, either this patient had suffered a very protracted course of COVID-19 or psychosis had started before infection.
Possible pathogenetic mechanisms were discussed in all case reports. Effector categories are the virus itself, inflammation caused by the infection, side effects of treatment, psychosocial stress and mere coincidence of psychosis with infection. The last possibility was least discussed and mostly discarded. In general, authors considered the infection with SARS-CoV-2 as causative for the psychosis in some form or another. Most authors had little doubt that the virus itself was responsible for the psychosis, even if there was no single proof of the virus having entered the brain.
Investigations of the CSF were done only in a minority of patients, and not always antigen tests in the CSF were performed. Only once, antibodies against SARS-CoV-2 could be detected (Noone et al., Reference Noone, Cabassa, Gardner, Schwartz, Alpert and Gabbay2020). By the way, this was also reported in another patient with mania, who was not included in this review for the lack of psychotic symptoms (Lu et al., Reference Lu, Wei, Jiang, Wu, Sheng, Zhou, Fang, Chen, Zheng, Chen, Liang and Hu2020).
This does not differ from the situation in neurology where CSF is analysed more frequently. Reviews on the neurological complications of SARS-CoV-2 infection reported proof of the virus’ presence in the brain in a small minority of cases only (Fotuhi et al., Reference Fotuhi, Mian, Meysami and Raji2020; Mohammadi et al., Reference Mohammadi, Moosaie and Aarabi2020; Najjar et al., Reference Najjar, Najjar, Chong, Pramanik, Kirsch, Kuzniecky, Pacia and Azhar2020; Travi et al., Reference Travi, Rossotti, Merli, D’amico, Chiappetta, Giussani, Panariello, Corradin, Vecchi, Raimondi, Baiguera, Nocita, Epis, Tarsia, Galbiati, Colombo, Fumagalli, Scaglione, Moreno, Percudani, Agostoni and Puoti2021) or not at all (Espíndola et al., Reference Espíndola, Siqueira, Soares, Lima, Leite, Araujo, Brandão and Silva2020; Frontera et al., Reference Frontera, Sabadia, Lalchan, Fang, Flusty, Millar-Vernetti, Snyder, Berger, Yang, Granger, Morgan, Patel, Gutman, Melmed, Agarwal, Bokhari, Andino, Valdes, Omari, Kvernland, Lillemoe, Chou, Mcnett, Helbok, Mainali, Fink, Robertson, Schober, Suarez, Ziai, Menon, Friedman, Friedman, Holmes, Huang, Thawani, Howard, Abou-Fayssal, Krieger, Lewis, Lord, Zhou, Kahn, Czeisler, Torres, Yaghi, Ishida, Scher, De Havenon, Placantonakis, Liu, Wisniewski, Troxel, Balcer and Galetta2021). It remains unclear whether this is a failure of testing techniques, caused by a rapid clearance of the virus from the brain (Espíndola et al., Reference Espíndola, Siqueira, Soares, Lima, Leite, Araujo, Brandão and Silva2020), or – more probable – depicts a subordinate role for the virus itself in neural damage (Fotuhi et al., Reference Fotuhi, Mian, Meysami and Raji2020). With time elapsing, it is becoming more and more clear that there is no big role for direct effects of the virus in the CNS (Johansson et al., Reference Johansson, Mohamed, Moulin and Schiöth2021; Lewis et al., Reference Lewis, Frontera, Placantonakis, Lighter, Galetta, Balcer and Melmed2021).
If not the virus itself, then inflammation could be causal: inflammation is considered as the predominant pathogenetic factor by most authors. ’Systemic inflammatory response syndrome’ (SIRS) describes the excessive reaction of the immune system to the infection with release of high amounts of cytokines and chemokines (Mohammadi et al., Reference Mohammadi, Moosaie and Aarabi2020), often also termed as ‘cytokine storm’. Binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2) receptors might contribute importantly to a pronounced inflammatory reaction (Verdecchia et al., Reference Verdecchia, Cavallini, Spanevello and Angeli2020). ACE2 physiologically degrades angiotensin-II to angiotensin 1–7. Beyond its effects on sodium and fluid regulation, angiotensin-II has strong proinflammatory effects by activating immune cell populations, elevating proinflammatory cytokines and generating intracellular free radicals (Benigni et al., Reference Benigni, Cassis and Remuzzi2010). Angiotensin 1–7 opposes these effects. Virus’ binding to ACE2 leads to an accumulation of angiotensin-II and therefore promotes inflammation (de Erausquin et al., Reference De Erausquin, Snyder, Carrillo, Hosseini, Brugha and Seshadri2021). The result is an upregulation of the cells of the innate immune system as monocytes, mast cells, macrophages and T-lymphocytes, with release of multiple proinflammatory cytokines. IL-6 and TNF-α are considered the main drivers of inflammation systemically and in the CNS (Medina-Enríquez et al., Reference Medina-Enríquez, Lopez-León, Carlos-Escalante, Aponte-Torres, Cuapio and Wegman-Ostrosky2020; Steardo et al., Reference Steardo, Steardo and Verkhratsky2020) and their levels correlate closely with severity of somatic symptoms and clinical outcome of COVID-19 (Jardim Vaz de Mello et al., Reference De Mello, Guimaraes Silva, Oliveira Correa Rabelo, Evaristo Leite, Vieira, Bahadori, Seifi and Godoy2020; Kempuraj et al., Reference Kempuraj, Selvakumar, Ahmed, Raikwar, Thangavel, Khan, Zaheer, Iyer, Burton, James and Zaheer2020; Raony et al., Reference Raony, De Figueiredo, Pandolfo, Giestal-De-Araujo, Oliveira-Silva Bomfim and Savino2020). The systemic inflammation reaches the brain in various ways, for example passage through areas without BBB, active transport of cytokines through the BBB, damaging the BBB or passage of activated immune cells through the BBB. Within the brain, inflammation mediators activate astrocytes and microglia as the brain’s immune system, which produce cytokines and chemokines on their part, causing neuroinflammation (Kempuraj et al., Reference Kempuraj, Selvakumar, Ahmed, Raikwar, Thangavel, Khan, Zaheer, Iyer, Burton, James and Zaheer2020; Mohammadi et al., Reference Mohammadi, Moosaie and Aarabi2020; Najjar et al., Reference Najjar, Najjar, Chong, Pramanik, Kirsch, Kuzniecky, Pacia and Azhar2020; Raony et al., Reference Raony, De Figueiredo, Pandolfo, Giestal-De-Araujo, Oliveira-Silva Bomfim and Savino2020). Hypoxia, which can be present even in mild forms of COVID-19, and stress are further drivers of neuroinflammation (Cole et al., Reference Cole, Capitanio, Chun, Arevalo, Ma and Cacioppo2015; Kempuraj et al., Reference Kempuraj, Selvakumar, Ahmed, Raikwar, Thangavel, Khan, Zaheer, Iyer, Burton, James and Zaheer2020; Steardo et al., Reference Steardo, Steardo and Verkhratsky2020). In brains of deceased patients after COVID-19, massive inflammatory alterations have been found, without viral presence (Yang et al., Reference Yang, Kern, Losada, Agam, Maat, Schmartz, Fehlmann, Stein, Schaum, Lee, Calcuttawala, Vest, Berdnik, Lu, Hahn, Gate, Mcnerney, Channappa, Cobos, Ludwig, Schulz-Schaeffer, Keller and Wyss-Coray2021).
Psychosis has been linked to inflammation in various aspects (Najjar et al., Reference Najjar, Pearlman, Alper, Najjar and Devinsky2013). Activation of microglia (leading to increased levels of proinflammatory cytokines) is considered to play a central role in the genesis of acute psychosis (De Picker et al., Reference De Picker, Morrens, Chance and Boche2017; Marques et al., Reference Marques, Ashok, Pillinger, Veronese, Turkheimer, Dazzan, Sommer and Howes2019). Neuroinflammation alters neurotransmission, disrupts neuronal connections and ultimately can lead to cell death (Kempuraj et al., Reference Kempuraj, Selvakumar, Ahmed, Raikwar, Thangavel, Khan, Zaheer, Iyer, Burton, James and Zaheer2020). As found in other viral infections and maybe more frequently than in other viruses (Travi et al., Reference Travi, Rossotti, Merli, D’amico, Chiappetta, Giussani, Panariello, Corradin, Vecchi, Raimondi, Baiguera, Nocita, Epis, Tarsia, Galbiati, Colombo, Fumagalli, Scaglione, Moreno, Percudani, Agostoni and Puoti2021), SARS-CoV-2 can induce para- or postinfectional processes which can lead to further psychiatric and neurologic syndromes (Kępińska et al., Reference Kępińska, Iyegbe, Vernon, Yolken, Murray and Pollak2020; Paterson et al., Reference Paterson, Brown, Benjamin, Nortley, Wiethoff, Bharucha, Jayaseelan, Kumar, Raftopoulos, Zambreanu, Vivekanandam, Khoo, Geraldes, Chinthapalli, Boyd, Tuzlali, Price, Christofi, Morrow, Mcnamara, Mcloughlin, Lim, Mehta, Levee, Keddie, Yong, Trip, Foulkes, Hotton, Miller, Everitt, Carswell, Davies, Yoong, Attwell, Sreedharan, Silber, Schott, Chandratheva, Perry, Simister, Checkley, Longley, Farmer, Carletti, Houlihan, Thom, Lunn, Spillane, Howard, Vincent, Werring, Hoskote, Jäger, Manji and Zandi2020). Based on structural similarities (Yapici-Eser et al., Reference Yapici-Eser, Koroglu, Oztop-Cakmak, Keskin, Gursoy and Gursoy-Ozdemir2021) of virus proteins with host proteins (‘molecular mimicry’), cross-reactive antibodies (Fotuhi et al., Reference Fotuhi, Mian, Meysami and Raji2020; Kreye et al., Reference Kreye, Reincke and Prüss2020) and T-cells acting against own tissues can cause neurologic (e.g. Guillain-Barre syndrome) as well as psychiatric syndromes (e.g. autoimmune psychosis) (Endres et al., Reference Endres, Maier, Leypoldt, Wandinger, Lennox, Pollak, Nickel, Maier, Feige, Domschke, Prüss, Bechter, Dersch and Tebartz Van Elst2020), which can be still present long after the virus has been cleared from the body (Kreye et al., Reference Kreye, Reincke and Prüss2020).
Concerning our sample of patients, we have to admit that signs of inflammation were rarely so severe that one would call it a ‘cytokine storm’. In half of the cases, somatic symptoms of COVID-19 were absent or did not necessitate inpatient treatment. About a quarter displayed no signs of inflammation and CRP was not elevated in 40%. Cytokines were only rarely assessed. Yet the lack of peripheral signs of inflammation does not preclude neuroinflammation. Many patients showed a time lag between somatic symptoms and start of psychosis so that peripheral inflammation possibly had abated at the time of psychiatric admission. Though it seems plausible that the risk for brain damage increases with intensity of inflammation, it is unclear what effect SARS-CoV-2 induced low-grade systemic inflammation has on the brain.
Possible side effects of therapeutics were frequently discussed. This report focusses on CS, CQ (only one patient) and HCQ administered before occurrence of psychosis, which happened in 30 (35.7%) patients. Yet in most of these cases, the beginning of psychosis was days till weeks after the end of administration so that a causal relationship was discarded by most authors. This was assumed only on eight occasions, concerning CS (five patients), CQ, HCQ, and the combination CS + HCQ (one each). Yet only in three patients (Benjelloun et al., Reference Benjelloun, Otheman and El Kettani2020; Grover et al., Reference Grover, Sahoo, Rijal and Mehra2021; Jiménez-Fernández et al., Reference Jiménez-Fernández, Solis, Martínez-Reyes, Alvarado-Dafonte, Soldado-Rodríguez and Rodríguez-Natal2021), the psychosis occurred during the treatment with the respective drug, in the rest 1–7 days later. How much this can contribute to occurrence of psychosis remains unclear. Even with incidence rates varying wildly, the risk for psychosis through treatment with CS is well established (Dubovsky et al., Reference Dubovsky, Arvikar, Stern and Axelrod2012; Ross & Cetas, Reference Ross and Cetas2012). ’Steroid psychosis’ is a broad term comprising severe neuropsychiatric complications, including depression, mania, delirium, and psychosis, affecting about 5% (range 2–50%) of patients treated with CS. The proportion of clear-cut psychosis among these amounts to about 15% (Lewis & Smith, Reference Lewis and Smith1983). The risk increases clearly with dosage and is highest during the first days of treatment, though occasionally cases at cessation of treatment have been described (Dubovsky et al., Reference Dubovsky, Arvikar, Stern and Axelrod2012). The intense discussion of HCQ as a cause for psychosis is contrasted by some recent papers, which prove the relative safety of HCQ. Even for CQ, which is generally more fraught with side effects due to a longer elimination half time and greater penetration through the BBB (Hamm & Rosenthal, Reference Hamm and Rosenthal2020; Kamat & Kumari, Reference Kamat and Kumari2021), an increased risk for psychosis (Papazisis et al., Reference Papazisis, Siafis, Cepatyte, Giannis, Stamoula, Tzachanis and Egberts2021) has not always been found (Sato et al., Reference Sato, Mano, Iwata and Toda2020), and analyses of various data bases found no increased risk for HCQ (Garcia et al., Reference Garcia, Revet, Yrondi, Rousseau, Degboe and Montastruc2020; Lane et al., Reference Lane, Weaver, Kostka, Duarte-Salles, Abrahao, Alghoul, Alser, Alshammari, Areia, Biedermann, Banda, Burn, Casajust, Fister, Hardin, Hester, Hripcsak, Kaas-Hansen, Khosla, Kolovos, Lynch, Makadia, Mehta, Morales, Morgan-Stewart, Mosseveld, Newby, Nyberg, Ostropolets, Woong Park, Prats-Uribe, Rao, Reich, Rijnbeek, Sena, Shoaibi, Spotnitz, Vignesh, Suchard, Vizcaya, Wen, De Wilde, Xie, You, Zhang, Lovestone, Ryan and Prieto-Alhambra2020; Edington et al., Reference Edington, Gadellha and Santiago2021; Papazisis et al., Reference Papazisis, Siafis, Cepatyte, Giannis, Stamoula, Tzachanis and Egberts2021). The warning regarding the risk of psychosis seems to be based on a handful of case reports which have accumulated during the long use of this drug (Emmanuel & Östlundh, Reference Emmanuel and Östlundh2020; Juurlink, Reference Juurlink2020).
Looking at patients without somatic symptoms of COVID-19 shows that the correlation of intensity of somatic symptoms with presentation of psychosis is poor: three patients were diagnosed as reactive (Jaworowski et al., Reference Jaworowski, Weiser, Gropp and Malka2020; Parra et al., Reference Parra, Juanes, Losada, Álvarez-Sesmero, Santana, Martí, Urricelqui and Rentero2020; Mollà Roig, Reference Mollà Roig2021), in two stress was present, but not considered decisive (Huarcaya-Victoria et al., Reference Huarcaya-Victoria, Meneses-Saco and Luna-Cuadros2020b). On the contrary, in four patients a ‘lack of preoccupation’ was documented and stress as a causal factor therefore excluded (Ferrando et al., Reference Ferrando, Klepacz, Lynch, Tavakkoli, Dornbush, Baharani, Smolin and Bartell2020) (three patients), (Alvarez-Cisneros et al., Reference Alvarez-Cisneros, Lara-Reyes and Sansón-Tinoco2021). One patient presented with a long-lasting manic psychosis (Russo et al., Reference Russo, Consoli, De Rosa, Calisi, Dono, Carrarini, Onofrj, De Angelis and Sensi2021), two with intense organic-like psychoses (in one patients with seizures) which led to a full diagnostic workup (Losee & Hanson, Reference Losee and Hanson2020; Elfil et al., Reference Elfil, Selby, Van Schooneveld and Fadul2021). One patient’s psychosis was complicated by adrenal insufficiency which afforded additional CS replacement (Spiegel et al., Reference Spiegel, Colangelo, Oplinger, Parkerson, Lamas, Cherukuru and Gill2021), and one patient developed a change of behaviour over 2 months, leading to a diagnosis of schizophreniform disorder (DeLisi, Reference Delisi2021). Thus, the lack of somatic symptoms does neither imply that psychosis presents with dominant reactive features, nor that it was short lived or less severe.
Also, the possibility that stressors caused the psychosis was discussed often but was considered decisive only in seven of the patients with infection. This against the background that there are those many reports of obviously stress-related psychoses that evolved in times of the pandemic without any infection. Maybe the importance of stressors has been underestimated given the presence of infection. On the other hand, the description of the cases with infection defers from the patients’ without infection as delusional themes concerning the pandemic were significantly less often reported in the first. This could be an argument for the far smaller importance of pandemic-induced stress in these patients but could also be due to a reporting bias in those patients whose psychosis was thought to be virus-induced from the start.
Both groups showed a very favourable course of psychosis. Psychotic symptoms developed in both groups very acutely within a few days in the vast majority of patients, in those with infection more often (88.5%) than in those without (59.6%). For both, the duration of psychosis was less than 2 weeks in more than half the patients, full remission was attained in about two-thirds of patients. In both groups, a fast response to treatment was frequently reported, in the group without infection more often (61.3%) than in the group with infection (39.3%). Karl Menninger’s paper (Menninger, Reference Menninger1919), describing cases of dementia praecox after the influanza pandemic of 1918, is frequently cited in these days and might foster concerns that the pandemic represents the starting point for new-onset schizophrenic disorders. Menninger himself had to revoke his diagnoses some years later (Menninger, Reference Menninger1994). From a clinical view, we have to underline that these first-episode ‘corona-psychoses’ have little in common with first-episode schizophrenia psychoses. Schizophrenia patients are very much younger and have a far longer prodromal phase. To appreciate the dramatically good outcome of these patients, a comparison with first-episode patients diagnosed with schizophrenia is helpful. In schizophrenia, only half of patients achieve a 50% reduction of psychopathological scores (PANSS or BPRS) (Zhu et al., Reference Zhu, Li, Huhn, Rothe, Krause, Bighelli, Schneider-Thoma and Leucht2017) and a review calculated the weighted mean of remission with 35.6% (AlAqeel & Margolese, Reference Alaqeel and Margolese2012). Age of onset also separates our patients from schizophrenia patients, because schizophrenia and schizophreniform disorder usually begin with a mean age of 25–29 years (Zarate et al., Reference Zarate, Tohen and Land2000; Boter et al., Reference Boter, Peuskens, Libiger, Fleischhacker, Davidson, Galderisi and Kahn2009; Segarra et al., Reference Segarra, Ojeda, Zabala, García, Catalán, Eguíluz and Gutiérrez2012; Kahn et al., Reference Kahn, Winter Van Rossum, Leucht, Mcguire, Lewis, Leboyer, Arango, Dazzan, Drake, Heres, Díaz-Caneja, Rujescu, Weiser, Galderisi, Glenthøj, Eijkemans, Fleischhacker, Kapur and Sommer2018), while our patients were about 20 years older. Description of Schneiderian first rank symptoms was rare, and negative symptoms were absent in these patients. Therefore, only three patients were diagnosed with schizophrenia or schizophreniform disorder. Of these, in two patients symptoms probably had already started prior to the pandemic, and only one patient can be considered as a potential manifestation of schizophreniform disorder after COVID-19.
According to DSM-5, late onset, absence of personal or family history of psychotic disorders, and visual hallucinations favour a diagnosis of PDGMC versus schizophrenia. Data on outcome and prognosis of PDGMC are scarce due to the wide variety of potential medical conditions, and DSM-5 states that the prognosis depends on the nature of the underlying somatic disorder. A paper summarising outcomes of psychosis due to chronic neurological illnesses still found outcomes and course better than in schizophrenia (Feinstein & Ron, Reference Feinstein and Ron1998). So far, we can state that the short-term outcome of psychoses concomitant with SARS-CoV-2 infection is very good but there is no information on the further fate of these patients. No follow-ups have been published until yet.
Limitations
We have done our best to collect a comprehensive compilation of case reports until December 2021. Case reports vary greatly in their comprehensiveness concerning symptomatology and treatment. On many occasions, it was not possible to distinguish between items not present or not reported. This might lead to bias, for example concerning the frequency of COVID-related delusions in patients with infections. Smith et al. (Reference Smith, Gilbert, Riordan, Helmke, Von Isenburg, Kincaid and Shirey2021) in their systematic review of cases with infection rated only one-third of reports with low risk of bias. Applying statistics to these data might be misleading for the lack of uniformity in reporting. Nevertheless, we took this path for the sake of clarity of presentation, but we must underline that all conclusions need to be considered with caution.
Reporting bias might be huge for many reasons. All our cases stem from hospitals or outpatient clinics, so patients not presenting there are not covered. We must assume that the psychopathology especially of very severe COVID-19 cases is less often reported when all efforts are centred to save the patient’s life. On the other hand, severe cases might be reported more frequently for heightened interest. Besides, it might be that ‘first’ cases have been reported more often during the course of the pandemic and later ones no more for lack of novelty. Some authors mentioned that they treated far more cases but selected only some for publication, for example (Deshpande & Livingstone, Reference Deshpande and Livingstone2021; Valdés-Florido et al., Reference Valdés-Florido, López-Díaz, Palermo-Zeballos, Garrido-Torres, Álvarez-Gil, Martínez-Molina, Martín-Gil, Ruiz-Ruiz, Mota-Molina, Algarín-Moriana, Guzmán-Del Castillo, Ruiz-Arcos, Gómez-Coronado, Galiano-Rus, Rosa-Ruiz, Prados-Ojeda, Gutierrez-Rojas, Crespo-Facorro and Ruiz-Veguilla2021).
Conclusion
New-onset psychosis in the context of SARS-CoV-2 infection is not rare, even if is not possible to determine an incidence rate. As our understanding of the pathophysiology of primary psychosis as schizophrenia is quite limited, our actual diagnostic systems classify psychosis following clinical experience and distinguish psychosis according assumed causal factors. Stress induced by the pandemic seems to be of major importance in the cases of psychosis without infection. Maybe the role of stress has been underestimated in the cases of psychosis with infection. Inflammation seems to be key for cases with infection yet there is poor concordance of the severity of peripheral inflammation with the occurrence of psychosis. Yet, the more severe the somatic symptoms of COVID-19, the more features of delirium may accompany psychosis. The mechanisms how and where the brain gets affected remain to be investigated. Research shows that processes of inflammation play a role as well in psychosis (Upthegrove et al., Reference Upthegrove, Manzanares-Teson and Barnes2014; Müller, Reference Müller2018; Momtazmanesh et al., Reference Momtazmanesh, Zare-Shahabadi and Rezaei2019; Misiak et al., Reference Misiak, Bartoli, Carrà, Stańczykiewicz, Gładka, Frydecka, Samochowiec, Jarosz, Hadryś and Miller2021) as in stress (Cole et al., Reference Cole, Capitanio, Chun, Arevalo, Ma and Cacioppo2015; Kempuraj et al., Reference Kempuraj, Selvakumar, Ahmed, Raikwar, Thangavel, Khan, Zaheer, Iyer, Burton, James and Zaheer2020; Steardo et al., Reference Steardo, Steardo and Verkhratsky2020). Thus, inflammation might be the unifying process linking infection, stress, and psychosis.
While we are still not able to depict a proper description of the biological mechanisms of psychosis, we are restricted to collect risk factors and it seems that SARS-CoV-2 is such a risk factor, in a biological as well in a psychological way. It would be interesting to learn about the further fates of the patients assembled in this paper but as for now no reports could be spotted. We hope that with increasing containment of the pandemic, a more systematic investigation of these cases will be possible. As proposed in the field of neurology (Podury et al., Reference Podury, Srivastava, Khan, Kakara, Tandon, Shrestha, Freeland, Wen and Sriwastava2021), it would be very helpful to create an international reporting system for such cases with standardised assessment tools concerning psychopathology, investigations (especially inflammation parameters) and long-term follow-up.










