1. Introduction
What makes us such peculiar animals? What is it about the human mind that has enabled us to transform our environments, to become so dependent on cooperation for survival, and thereby to construct the edifices of knowledge and skill in which our lives are embedded: craft, technology, agriculture, science, religion, law, politics, history, music, trade, art, literature, and sport? Contemporary answers assume that adult humans have mental faculties different from those of all other extant animals, and the differences have two sources: nature and nurture. Whether distinctively human faculties are understood to be symbolic or sub-symbolic, model-based or model-free, general- or special-purpose, modular or holistic, optimal or kluge-ridden, it is assumed that, insofar as they do their jobs well, it is because these faculties have been shaped by natural selection operating on genetic variants (nature) and by interaction between the neurocognitive system and its environment in the course of an individual's development (nurture).
Cognitive Gadgets: The Cultural Evolution of Thinking (Heyes Reference Heyes2018; henceforth Cognitive Gadgets) argues that the most strikingly distinctive features of the human mind come from a third source: culture. Natural selection operating on cultural variants – traits inherited through social interaction – doesn't only give us beliefs, tools and techniques; it also produces new neurocognitive mechanisms. In a slogan: Cultural evolution shapes not just what we think but how we think it. In a saintly metaphor: Cultural evolution changes not only the grist but also the mills of the human mind (Aquinas Reference Aquinas1272; Heyes Reference Heyes2012a). In a contrapuntal catchphrase: Distinctively human cognitive mechanisms – such as language, theory of mind, causal reasoning, episodic memory, imitation, and morality – are not “cognitive instincts” (Pinker Reference Pinker1994) but “cognitive gadgets.” These mechanisms, which are absent or merely nascent in other animals, were not designed by human minds, but they are the products of human rather than genetic agency. They are gadget-like in being relatively small but crucially important parts of the mind. The bulk of our behaviour is controlled by mechanisms we share with other animals, but cognitive gadgets are what make human minds and lives so very odd.
Literacy is a cognitive gadget. The capacity to read printed matter depends on dedicated neurocognitive mechanisms. Written language emerged only 5,000 to 6,000 years ago, too recently in human history for the genetic evolution of neurocognitive mechanisms specialised for reading. Therefore, insofar as those mechanisms do their jobs well, it must be because they have been shaped by cultural evolution.
Cognitive Gadgets is an academic book written by a psychologist for accessibility to psychologists, neuroscientists, evolutionary biologists, anthropologists, archaeologists, computer scientists, economists, philosophers, and others interested in human evolution. I worked hard to make it short, hoping it would be read even in disciplines in which books are rare beasts. One of the consequences of brevity is that the logical geography in chapter 1 is local. Focussing on closely related ideas in the recent past, chapter 1 identifies the framework developed in the book as “cultural evolutionary psychology,” a direct descendant of “evolutionary psychology” (Barkow et al. Reference Barkow, Cosmides and Tooby1992; Pinker Reference Pinker1994) and “cultural evolutionary theory” (Boyd & Richerson Reference Boyd and Richerson1985; Campbell Reference Campbell1965; Cavalli-Sforza & Feldman Reference Cavalli-Sforza and Feldman1981; Dennett Reference Dennett1990; Reference Dennett1991; Henrich Reference Henrich2015) (see Fig. 1). Cultural evolutionary psychology is like evolutionary psychology in having the human mind as its explanatory target, and like cultural evolutionary theory in emphasising the importance of social learning as a force in human evolution, but it differs from both of these approaches in suggesting that distinctively human cognitive mechanisms get their adaptive characteristics from cultural rather than genetic evolution.
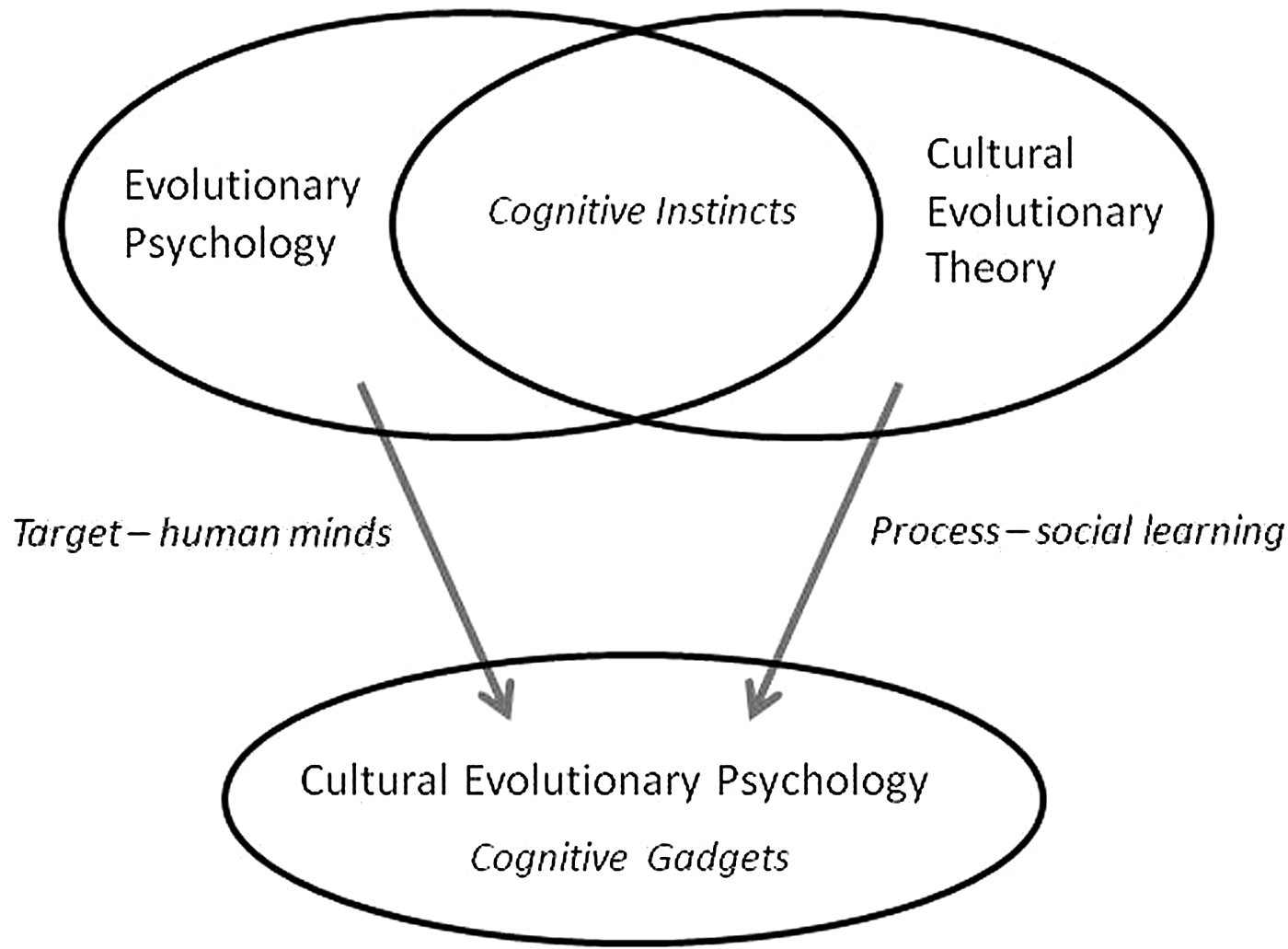
Figure 1. Relations between evolutionary psychology, cultural evolutionary theory, and cultural evolutionary psychology.
Viewed more broadly and with greater historical depth, the central thesis of Cognitive Gadgets addresses the modularity debate in cognitive science (Fodor Reference Fodor1983; Samuels Reference Samuels2012) and discussions of functional specialisation in ethology (de Waal & Ferrari Reference De Waal and Ferrari2010; Lorenz Reference Lorenz and Pribram1969). It suggests that, at least in humans, specialised cognitive mechanisms are built by general-purpose cognitive mechanisms; modules are acquired (Karmiloff-Smith Reference Karmiloff-Smith1995). In making this case, Cognitive Gadgets joins the battle initiated by the British Empiricists 300 years ago over the power of general-purpose mechanisms of learning, siding with advocates of deep learning, predictive coding, hierarchical reinforcement learning, causal modelling, and Bayesians of almost every stripe (Lake et al. Reference Lake, Ullman, Tenenbaum and Gershman2017). It also challenges the use of the behavioural gambit in economics and behavioural ecology (Fawcett et al. Reference Fawcett, Hamblin and Giraldeau2012; Nettle et al. Reference Nettle, Gibson, Lawson and Sear2013), discouraging a black box approach to neurocognitive mechanisms (Heyes Reference Heyes2016a), and builds on research in developmental psychology and elsewhere documenting the importance of cultural learning and cross-cultural variation in the way minds work (Haun et al. Reference Haun, Rapold, Cal, Janzen and Levinson2006; Legare & Nielson Reference Legare and Nielsen2015; Nisbett Reference Nisbett2010; Shiraev & Levy Reference Shiraev and Levy2014; Tomasello Reference Tomasello1999). At the broadest level, in common with evo-devo (West-Eberhard Reference West-Eberhard2003; Reference West-Eberhard2005), and the extended evolutionary synthesis (Laland et al. Reference Laland, Uller, Feldman, Sterelny, Müller, Moczek, Jablonka and Odling-Smee2015), Cognitive Gadgets stresses the critical, formative roles of developmental processes and environmental factors in the emergence of human cognition.
Cognitive Gadgets has four foundational chapters (1–4), four case study chapters (4–8) each focusing on one cognitive gadget, and a concluding chapter (9).
2. Nature, nurture, culture
2.1. Biological information
The development of every aspect of human behaviour and cognition, like the development of all biological systems, depends on a rich, turbulent stew of factors. There are no pure cases of nature or of nurture; no biological characteristic is caused only by “the genes” or only by “the environment.” Nonetheless, drawing on the teleosemantic conception of information (Millikan Reference Millikan1984; Shea Reference Shea2013), I argue in chapter 2 that psychologists and biologists can and should seek to isolate the contributions of nature (genetically inherited information), nurture (information derived from direct interaction between the developing system and its environment), and culture (information inherited via social interaction) to human cognitive development. Without this purpose and discipline, there is a risk that explanations of cognitive development will be no more than unwieldy descriptions, like Lewis Carroll's fictional map with a scale of one mile to one mile, or manageable only because they privilege some causes over others in an arbitrary way. Arbitrary privilege dominated the behavioural sciences of the twentieth century. As the pendulum swung from instinct theory (Kuo Reference Kuo1922) to behaviourism (Watson Reference Watson1930) and back again to evolutionary psychology via classical ethology (Lorenz Reference Lorenz1965; Tinbergen Reference Tinbergen1963) and sociobiology (Wilson Reference Wilson1975), researchers fixated on nature, then on nurture, and finally put the genes back in the ascendant.
2.2. Cultural evolution
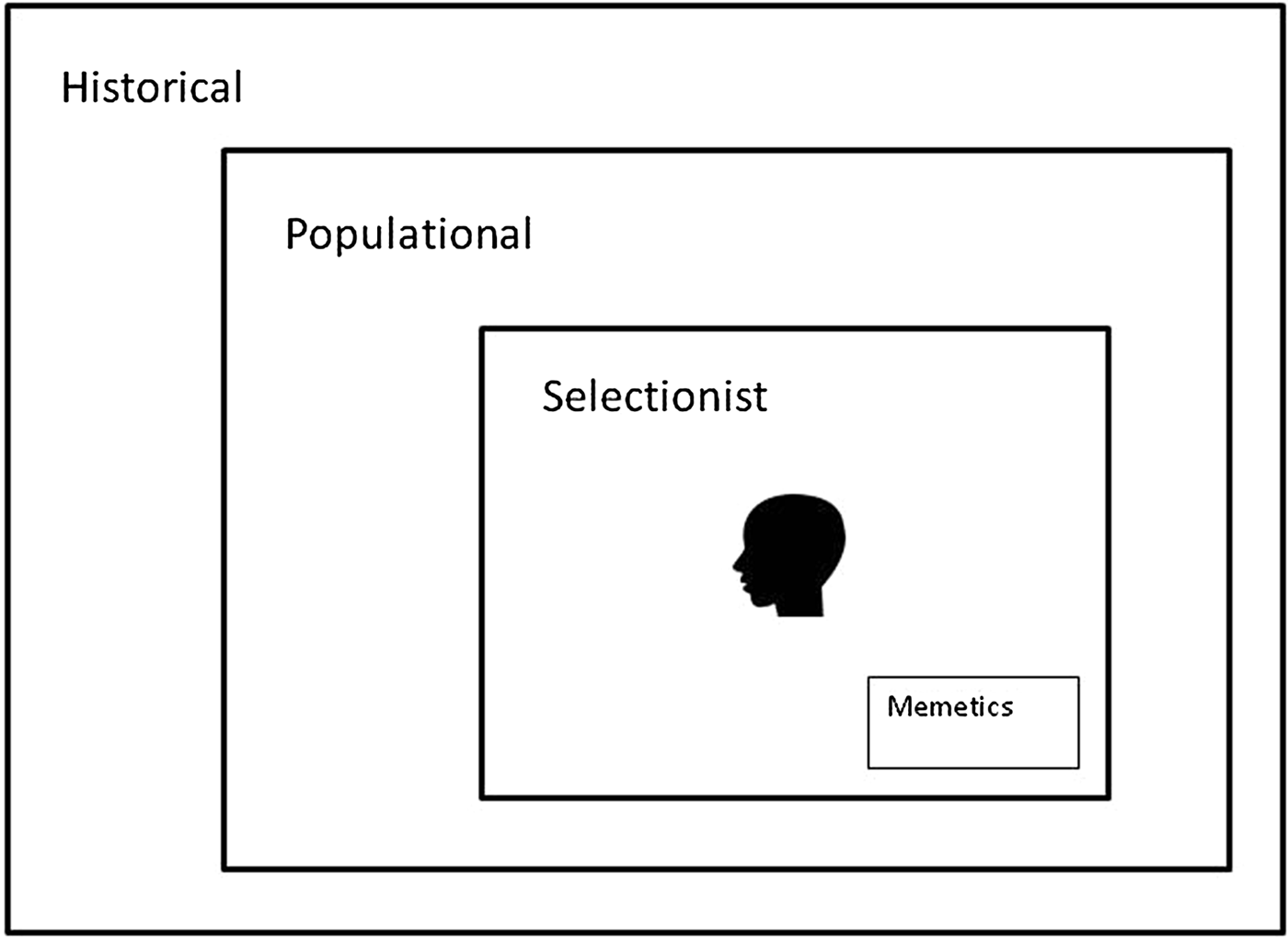
The importance of culture (sensu information inherited via social interaction) in shaping human behaviour has been emphasised by cultural evolutionists with increasing force since the 1980s (Boyd & Richerson Reference Boyd and Richerson1985; Campbell Reference Campbell1965; Cavalli-Sforza & Feldman Reference Cavalli-Sforza and Feldman1981; Henrich Reference Henrich2015; Morin Reference Morin2015; Sperber Reference Sperber1996). The idea of cultural evolution comes in three strengths: historical, populational, and selectionist (Fig. 2; Brusse Reference Brusse2017; Godfrey-Smith Reference Godfrey-Smith2009; Lewens Reference Lewens2015). When the term cultural evolution is used in the weakest historical sense, it means nothing more than change over time in some characteristic that varies between human groups. The stronger populational conception assumes that large-scale changes of this sort (e.g., changes in the distribution within a population of the use of particular technologies, or the consumption of certain foods) are the aggregate consequences of many episodes of social learning – of episodes in which individuals learn from others to use a particular technology or to eat a certain food. The strongest conception of cultural evolution, the selectionist view, shares the populational assumption and claims that the conditions necessary for Darwinian or natural selection are present in the cultural domain: There are mechanisms for introducing variation, selection processes, and mechanisms preserving selected variants (Campbell Reference Campbell and Schlipp1974). Cognitive Gadgets pursues the selectionist approach because this approach has the potential to explain the adaptive character of distinctively human cognition mechanisms – why they do their jobs reasonably well. It assumes that genetic evolution and cultural evolution are based on the same variation-and-selective-retention heuristic, and proposes that, rather than being on a short “genetic leash” (Lumsden & Wilson Reference Lumsden and Wilson2005, p. 144), cultural evolution is highly autonomous with respect to genetic evolution.
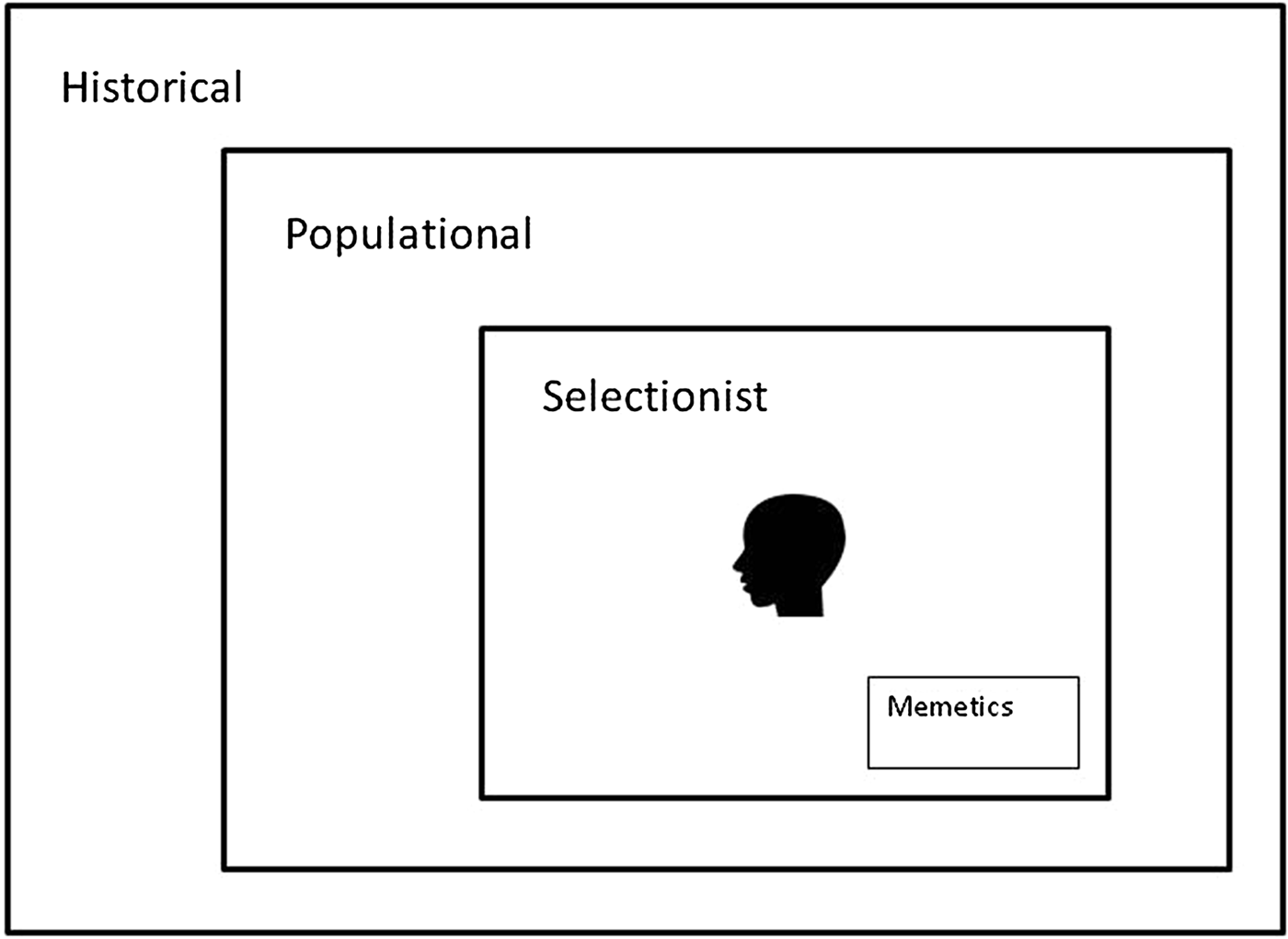
Figure 2. Relations between purely historical, populational, and selectionist conceptions of cultural evolution.
2.3. Cultural evolution of cognitive mechanisms
To apply a selectionist view of cultural evolution not only to beliefs and behaviour (the grist of the mind) but also to cognitive mechanisms (the mills), it is necessary to identify variants, routes of inheritance, and mechanisms of inheritance.
2.3.1. Variants
Variants, or traits, are the things to be quantified in calculations of fitness. In the case of mental grist, it is difficult to isolate variants in a principled way, because the only guide is folk psychology. We are forced to consult common sense or intuition for hypotheses about where one belief ends and another begins – about whether a practice, such as eating spicy food, constitutes one behaviour or many. In contrast, cognitive science is a rich source of empirically grounded hypotheses about variant cognitive mechanisms (mills). It stipulates that there is only one token of each type of cognitive mechanism in each brain and distinguishes types of cognitive mechanisms in a functional way, according to the kind of information it can process and the computations and representations it uses to process the information. For example, in the dual-route cascade model of reading (Coltheart et al. Reference Coltheart, Rastle, Perry, Langdon and Ziegler2001), a “reading aloud mechanism” is defined as a mechanism that can convert script into speech, and one reading aloud mechanism can differ from another in terms of the range of script sequences it can convert into speech (only regular, or regular and irregular words) and the types of representations (sensory and/or structured) it uses.
2.3.2. Routes of inheritance
The cultural inheritance of cognitive mechanisms, like that of beliefs and behaviour, can be vertical (from biological parents to their offspring), oblique (from individuals of one biological generation to genetically unrelated or distantly related individuals of the next generation), and/or horizontal (between individuals of the same biological generation) (Cavalli-Sforza & Feldman Reference Cavalli-Sforza and Feldman1981). The importance of each route may vary across cultures and types of cognitive mechanism, but a distributed pattern is likely to be common, in which all three routes see heavy traffic at different times in development. For example, in contemporary Western societies, the foundations of mindreading – the capacity to ascribe thoughts and feelings – are laid in early childhood through interaction with parents (vertical; Slaughter & Peterson Reference Slaughter, Peterson, Siegal and Surian2012) and other members of the parental generation (oblique; Lewis et al. Reference Lewis, Freeman, Kyriakidou, Maridaki-Kassotaki and Berridge1996). Later, when children and adults talk to one another about people's motivations and misapprehensions and read literary fiction, the development of mindreading is influenced predominantly by peers (horizontal; Kidd & Castano Reference Kidd and Castano2013).
2.3.3. Mechanisms of inheritance
It is risky to use words like “copying” and “transmission” to describe any mechanism of cultural inheritance. The processes that send beliefs and behaviour along the vertical, oblique, and horizontal routes are seldom analogous to DNA replication (Heyes Reference Heyes2017a), and a cognitive mechanism is certainly not a pellet of information that can be copied inside your head, sent through the air, and planted wholesale in my head. Rather, cognitive mechanisms are culturally inherited through social interactions, sometimes with many agents over an extended period of developmental time; these interactions gradually shape a child's cognitive mechanisms so that they resemble those of the people around them. Reading is a clear example. Everyone agrees that children are typically taught to read, that literacy training produces new neurocognitive mechanisms, and that we do not genetically inherit specific predispositions to develop these mechanisms. Cultural evolutionary psychology merely draws attention to the fact that literacy training is a set of social interactions that provide demonstrations, instructions, feedback, and encouragement in formal and informal settings. If literacy training were achieved by planting a “reading chip” in each child's brain, the cultural inheritance of reading would be more like the genetic inheritance of eye colour, but it would not necessarily be more effective in preserving selected variants.
2.4. Nature, nurture, culture – In practice
The final section of chapter 2 turns to a practical question: By what empirical methods can we tease apart the contributions of nature, nurture, and culture to the development of cognitive mechanisms? I argue that the methods required are means of distinguishing “poverty of the stimulus” (Chomsky Reference Chomsky1965) from “wealth of the stimulus” (Ray & Heyes Reference Ray and Heyes2011) – cases in which the developmental environment provides too little (poverty) or at least enough (wealth) usable information to explain the properties of a cognitive mechanism. Poverty is a sign that the development of an adaptive cognitive trait depends on genetically inherited information (nature), whereas wealth is a sign that development depends on learning in a broad sense (nurture) and/or on culturally inherited information (culture). Where there is wealth, nurture is indicated when cognitive development varies with features of the environment in which development is actually occurring, with information that can be acquired by asocial learning, and by the kinds of social learning found in a broad range of animals. Culture is indicated when cognitive development varies with longer-term features of the environment: features that may not be present when a particular individual is developing or that can be acquired only via the kinds of social learning known as cultural learning.
Training studies can help distinguish the roles of nature, nurture, and culture (e.g., De Klerk et al. Reference de Klerk, Johnson, Heyes and Southgate2015; Lohmann & Tomasello Reference Lohmann and Tomasello2003), but most of the empirical methods with the power to parse cognitive development examine patterns of spontaneous covariation. They relate differences in cognitive ability to opportunities for learning and social learning across (1) time points in development, (2) groups or individuals within a human population, (3) human populations, or (4) species. Examples of these methods are found in developmental psychology, cognitive psychology, cognitive neuroscience, behavioural genetics, cross-cultural psychology, and ethology, but there is currently a tendency in all of these fields to document cognitive variation without asking where it comes from, or laced with the assumption that nature is the dominant force.
3. Starter kit
Cognitive Gadgets suggests that the genetic starter kit for human cognition, although extensive, is very similar to the starter kits of other animals, including chimpanzees. In the course of hominin evolution, natural selection operating on genetic variants tweaked the mind in small but important ways. Genetic evolution has not given us programmes for the development of powerful domain-specific cognitive mechanisms, such as mindreading and language, but it has made us friendlier than our primate ancestors; enhanced our attentional biases towards other agents; and expanded our capacities for domain-general learning and executive control. These are the “Small Ordinary” gene-based changes that enable developing humans to upload “Big Special” cognitive mechanisms – cognitive gadgets – from their culture-soaked environments (Heyes Reference Heyes2018, pp. 52–53).
3.1. Emotion and motivation
There is evidence that modern humans are more socially tolerant (less aggressive to conspecifics) and more socially motivated (more inclined to seek and value social rewards) than our primate ancestors, and that these propensities are due to genetic evolution. Some of the most striking evidence of heightened social tolerance comes from archaeological work showing that, in the last 200,000 years, human skulls have undergone “craniofacial feminization” (Cieri et al. Reference Cieri, Churchill, Franciscus, Tan, Hare, Athreya, Holliday, Nowell, Steele, Weaver and Wrangham2014). Combined with studies of domestication in a range of nonhuman species, including wolves (Darwin Reference Darwin1868; Wilkins et al. Reference Wilkins, Wrangham and Fitch2014), these craniofacial changes suggest a reduction in androgen activity favoured by genetic evolution because it made humans less likely to initiate and elicit aggression from conspecifics. In the case of social motivation, there are signs that humans have an exaggerated, inborn tendency to enjoy “response-contingent stimulation” – events, typically social in origin, that are predicted or controlled by their own actions (Floccia et al. Reference Floccia, Christophe and Bertoncini1997). This may be due to upregulation of oxytocin, a neuropeptide that has been tweaked by genetic evolution in numerous ways over the last 700 million years (Roney Reference Roney2016).
Increments in social tolerance and motivation are quantitative changes in temperament, not the kind of thing one would normally expect to support a cognitive revolution. But they are important because they give developing humans access to a wide range of teachers and expert models, not only mothers, and incline them to act and think in any way that yields social rewards.
3.2. Attention
Social tolerance and motivation get developing humans up close and personal with a wide range of people who are equipped to fill and shape their minds with culturally inherited information. Genetically inherited input biases ensure that, from birth, human children target their attention on these experts, ready to drink in the information they have to offer (Heyes Reference Heyes2003).
In common with many other animals, human newborns attend more to biological than nonbiological motion (Bardi et al. Reference Bardi, Regolin and Simion2011; Reference Bardi, Regolin and Simion2014). Unlike other primate species, we also have inborn preferences for faces and voices. At birth, human babies turn their heads for longer to track a face-like triangle of dark blobs than an inversion of the same stimulus (Johnson et al. Reference Johnson, Dziurawiec, Ellis and Morton1991; Reid et al. Reference Reid, Dunn, Young, Amu, Donovan and Reissland2017). They also suck harder to hear speech sounds than synthetic sounds with similar pitch contour and spectral properties (Vouloumanos & Werker Reference Vouloumanos and Werker2007). In the first year of life, both of these attentional biases become more specific. For example, the neonatal “blob bias” becomes a preference for human over other primate faces at three months (Dupierrix et al. Reference Dupierrix, de Boisferon, Méary, Lee, Quinn, Di Giorgio, Simion, Tomonaga and Pascalis2014), and for human faces making direct eye contact at four months (Vecera & Johnson Reference Vecera and Johnson1995). Gaze-cuing, a tendency to direct attention to the object or area in front of moving eyes, appears at two to four months (Hood et al. Reference Hood, Willen and Driver1998). At between 6 and 12 months, gaze-cuing becomes more selective and active: Infants become more inclined to follow gaze when a gaze shift is preceded by direct eye contact (Senju & Csibra Reference Senju and Csibra2008), and to look back and forth between an adult's face and an object to check that they have the right spatial target (Carpenter & Call Reference Carpenter, Call, Metcalf and Terrace2013).
Each stage in this developmental sequence makes infants more teachable by increasing the extent to which their attention is controlled by knowledgeable adults. Some researchers see a number of genetic adaptations coming online in the course of the sequence, including mindreading, but in Cognitive Gadgets, I argue – using the parsing methods outlined in chapter 2 – that there is no compelling evidence for this view. As long as social rewards are more likely to follow direct eye contact than a glimpse of averted gaze, and as long as gaze shifts after eye contact better predict an encounter with an interesting object, reinforcement learning can build the full panoply of gaze-cuing phenomena on the foundation of a simple, genetically inherited face preference (Moore & Corkum Reference Moore and Corkum1994; Paulus et al. Reference Paulus, Hunnius, Vissers and Bekkering2011; Triesch et al. Reference Triesch, Teuscher, Deák and Carlson2006).
3.3. Cognition
Associative learning is a set of domain-general processes, including stimulus-stimulus and reinforcement learning, that have been investigated using Pavlovian and instrumental conditioning procedures (Pearce Reference Pearce2013). Associative learning has been found in every vertebrate and invertebrate group where it has been sought, and in a wide range of functional contexts from foraging to predator avoidance, mate choice, and navigation (Heyes Reference Heyes2012b; MacPhail Reference MacPhail1982; Shettleworth Reference Shettleworth2010). Comparisons across extant species suggest that genetic evolution has made some qualitative changes to associative learning in the course of its multi-million-year history, fashioning it into a powerful method of tracking causal/predictive relationships between events (Dickinson Reference Dickinson2012). There is no evidence that associative learning has undergone major, qualitative changes in the recent past and certainly not in the hominin line, but it is likely that, compared with other apes, we are genetically prepared to forge associations faster, learn more of them in parallel, and to attach associations to specific contexts more readily (Fagot & Cook Reference Fagot and Cook2006; Holland Reference Holland1992).
When associative learning was thought to control nothing more than “spit and twitches” (Rescorla Reference Rescorla1988), our expanded capacity for this kind of domain-general learning seemed to have nothing to do with the peculiarity of human lives. However, recent work, much of it social cognitive neuroscience, indicates that associative learning plays a critical role in our capacities to teach and engage in group decision-making. For example, associative learning enables us to keep track of the relationship between a pupil's actions and their outcomes (Apps et al. Reference Apps, Lesage and Ramnani2015), and to weigh advice from another agent against our own experience (Behrens et al. Reference Behrens, Hunt, Woolrich and Rushworth2008; Garvert et al. Reference Garvert, Moutoussis, Kurth-Nelson, Behrens and Dolan2015).
Although not as phylogenetically widespread as associative learning, executive functions – inhibitory control, working memory, and cognitive flexibility – are also found in a range of species (Cook et al. Reference Cook, Brown and Riley1985; MacLean et al. Reference MacLean, Hare, Nunn, Addessi, Amici, Anderson, Aureli, Baker, Bania, Barnard, Boogert, Brannon, Bray, Bray, Brent, Burkart, Call, Cantlon, Cheke, Clayton, Delgado, DiVincenti, Fujita, Herrmann, Hiramatsu, Jacobs, Jordan, Laude, Leimgruber, Messer, Moura, Ostojić, Picard, Platt, Plotnik, Range, Reader, Reddy, Sandel, Santos, Schumann, Seed, Sewall, Shaw, Slocombe, Su, Takimoto, Tan, Tao, van Schaik, Virányi, Vesalberghi, Wade, Watanabe, Widness, Young, Zentall and Zhao2014; Matzel & Kolata Reference Matzel and Kolata2010). No one doubts that executive functions play a major role in human cognition, that they are more highly developed in humans than in other animals, or that a good deal of this expansion is due to nurture and culture (Diamond Reference Diamond2013). However, there is reason to believe that genetic evolution – nature – has also played a part in expanding the power, capacity, and agility of executive function. The most widely cited evidence comes from neuroanatomical studies showing that the prefrontal cortex, which is focally involved in executive function, is disproportionately larger (Passingham Reference Passingham2008; Passingham & Smaers Reference Passingham and Smaers2014; Rilling Reference Rilling2014) and more extensively connected with phylogenetically older brain areas (Anderson & Finlay Reference Anderson and Finlay2014; Peterson & Posner Reference Petersen and Posner2012; Zilles Reference Zilles, Dehaene, Duhamel, Hauser and Rizzolatti2005) in humans than in chimpanzees.
4. Cultural learning
Cultural evolutionary psychology is both a framework for research and a hypothesis. As a framework, it recognises that distinctively human cognitive mechanisms can be shaped by culturally inherited information, as well as by genetically inherited information and learning (chapter 2). As a hypothesis, it proposes that cultural inheritance has played the dominant role in shaping all or most distinctively human cognitive mechanisms. To advance the hypothesis, chapters 5–8 each look in detail at evidence relating to one type of distinctively human cognition: selective social learning, imitation, mindreading, and language. These are all varieties of cultural learning.
Cultural learning is especially important for two reasons. First, both evolutionary psychologists and cultural evolutionary theorists, although divided on many issues, are united in assuming that the mechanisms of cultural learning are genetically inherited. Therefore, cultural evolutionary psychology warrants pursuit as a descendant of evolutionary psychology and cultural evolutionary theory only if there are good reasons to challenge this consensus. Second, from the perspective of cultural evolutionary theory, which I broadly share, mechanisms of cultural learning play a crucial role in making human lives so different from those of other animals. Like other distinctively human faculties, cultural learning meets challenges that arise in an individual's lifetime, enabling each of us to navigate the world of people (cf. face processing) and things (cf. causal understanding). However, unlike other faculties, cultural learning also underwrites a whole new inheritance system: cultural evolution. It is a gift that goes on giving. Cultural learning enables each person and social group to benefit from the accumulated experience of innumerable other people, past and present, and thereby collectively to acquire knowledge and to develop skills that are way beyond those of other species.
Cultural learning is typically understood to be a subset of processes known as social learning, and social learning processes are thought to overlap with those of asocial or individual learning (e.g., Henrich Reference Henrich2015). This way of thinking (shown in Fig. 3) has been shaped by the anthropologists, biologists, economists, and mathematicians who have pioneered research on cultural evolution, and it has done some good service. However, from a cognitive science perspective, the framework in Figure 3 has two significant problems:
1. Cultural evolutionists tend to treat as processes phenomena that cognitive scientists would regard as effects, that is, as things to be explained rather than things that do the explaining. They ignore the cognitive and neurological processes that produce observable changes in behaviour (e.g., Whiten & Ham Reference Whiten and Ham1992).
2. Cultural learning is understood to be a “sophisticated subclass of social learning” (Henrich Reference Henrich2015, p. 13), but there are no ground rules, empirical or conceptual, for deciding whether a particular type or example of social learning is or is not an example of cultural learning.
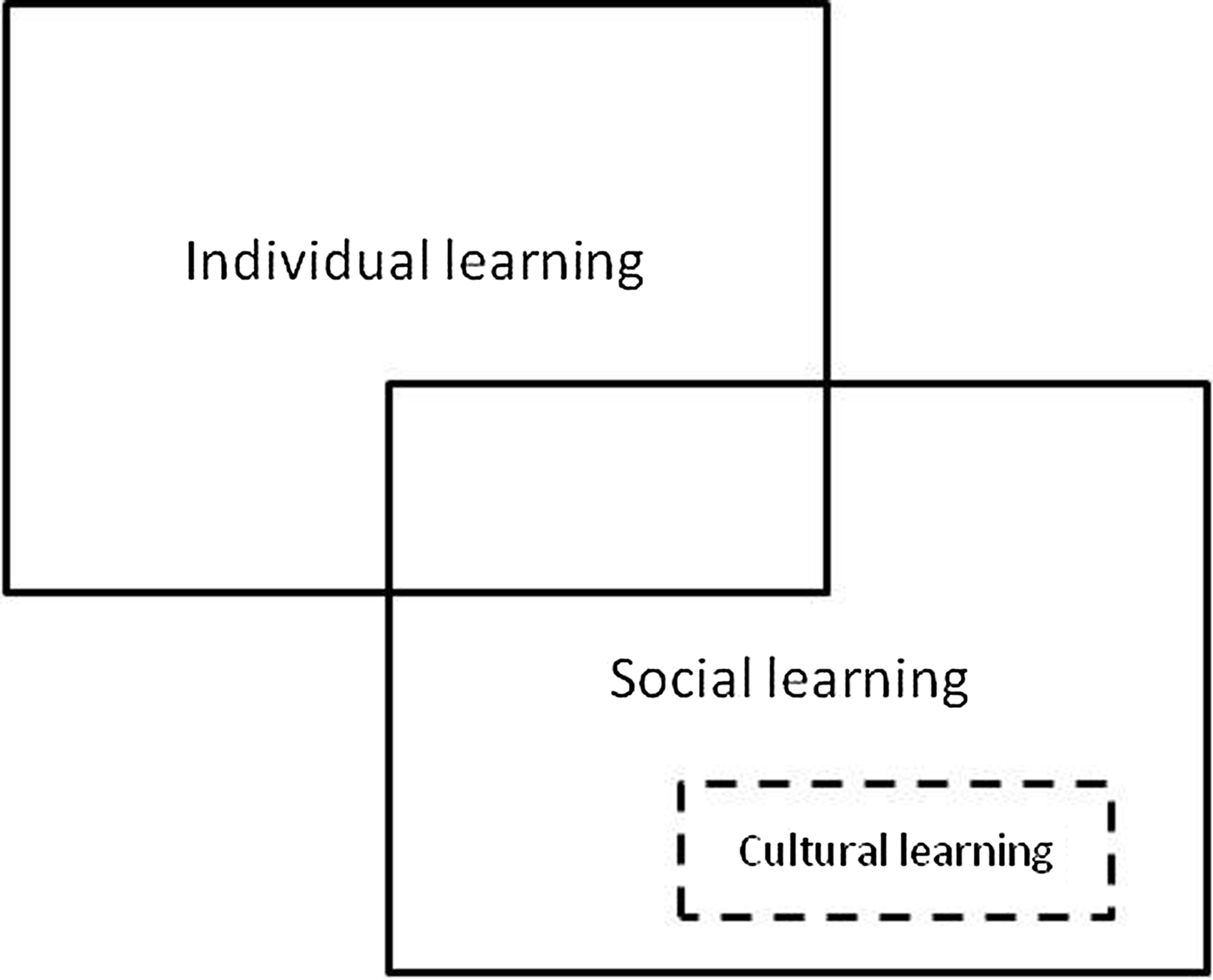
Figure 3. The received view of relations between individual learning, social learning, and cultural learning.
To enable dialogue between cultural evolutionary theory and cognitive science (Heyes Reference Heyes2017b), I propose a subtly different way of situating cultural learning, shown in Figure 4. In this alternative framework, the superordinate category is learning – encoding for long-term storage information acquired through experience. When learning is assisted by contact with other agents, it is called social learning. When learning is not assisted by other agents, it is called asocial learning or individual learning. Cultural learning is a subset of social learning involving cognitive processes that are specialised for cultural evolution – for example, processes that enhance the fidelity with which information is passed from one agent to another. This framework does not allude to processes in distinguishing asocial from social learning, and therefore avoids the misleading impression that social learning is known to depend on different cognitive mechanisms from asocial learning. Furthermore, although it makes the conventional assumption that cultural learning involves processes specialised for cultural inheritance, it does not embody any assumptions about how or why these processes are specialised. Rather, it is a framework for investigation of three questions that cultural evolutionists rarely tackle:
1. Cognition question: How do the mechanisms of cultural learning differ from those of social learning at the cognitive level?
2. Specialisation question: How have genetic evolution and/or cultural evolution contributed to the specialisation of cultural learning?
3. Contribution question: In what ways do the features that distinguish cultural learning from social learning contribute to cultural inheritance? For example, do they make “improved” cultural variants more likely than “unimproved” variants to be passed on?
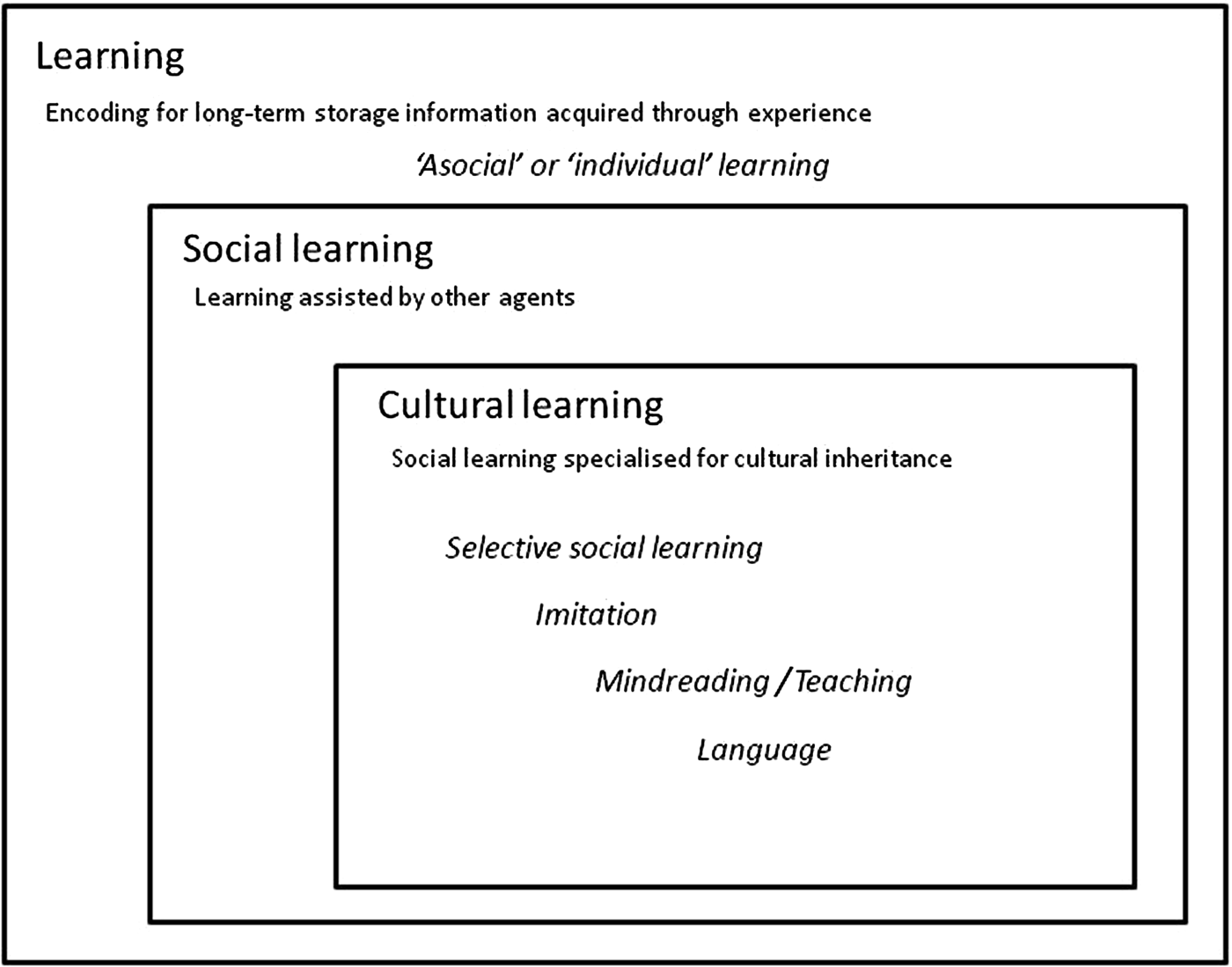
Figure 4. A framework for research on the relations between learning, social learning, and cultural learning, enabling dialogue between cognitive science and cultural evolutionary theory.
Rather than appealing to sophistication, implying that we already know what is distinctive about the mechanisms of cultural learning at the cognitive level (question 1 above), this framework defines cultural learning by ostension – by pointing at putative examples of cultural learning. The cultural learning box in Figure 4 lists the five categories of psychological phenomena (each containing behavioural effects and weakly specified cognitive processes) most commonly said by cultural evolutionists to be types of cultural learning:
1. Selective social learning (also known as learning biases, transmission biases, social learning rules, and social learning strategies)
2. Imitation (called true imitation when imitation is used as a synonym for social learning)
3. Teaching (or pedagogy)
4. Mindreading (also called theory of mind, mentalising, shared intentionality, folk psychology, and social understanding)
5. Language (so good they named it once)
These five categories are a natural place to start asking the cognition, specialisation, and contribution questions about cultural learning.
5. Selective social learning
In both of the schemes shown in Figures 3 and 4, social learning names a rag bag of behavioural effects – from a snail following a slime trail, to a student reading about calculus – in which learning by one agent, the “observer,” is influenced in some way by contact of some sort with another agent, the “model” or “demonstrator.” Social learning is said to be selective primarily when the influence of the model varies with the circumstances of the encounter (“when” selectivity; e.g., greater influence when the observer's environment has recently changed, known as a copy when uncertain social learning strategy), or with some feature of the available models (“who” selectivity; e.g., greater influence by older than younger models, known as a copy older individuals social learning strategy).
Selective social learning has been a focus of cultural evolutionary studies since the 1980s, but it barely appears on the radar of cognitive scientists. Consequently, whereas chapters 6–8 bring cultural evolutionary theory to bear on problems in cognitive science, chapter 5 brings cognitive science into closer contact with cultural evolutionary theory. More specifically, chapter 5 tackles head-on the cognition, specialisation, and contribution questions.
Addressing the cognition question, I suggest that most social learning is (1) mediated by the same domain-general, associative processes as asocial learning, and (2) made selective by the same broadly attentional processes that make asocial learning selective. Four lines of evidence support the first of these hypotheses (Heyes Reference Heyes1994; Reference Heyes2012c):
1. Social and asocial learning ability covary. Among birds and primates, species and individuals that perform well in tests of social learning tend also to perform well in tests of asocial learning (Boogert et al. Reference Boogert, Giraldeau and Lefebvre2008; Bouchard et al. Reference Bouchard, Goodyer and Lefebvre2007; Reader et al. Reference Reader, Hager and Laland2011).
2. Solitary animals are capable of social learning. In laboratory tests, animals such as red-footed tortoises (Wilkinson et al. Reference Wilkinson, Kuenstner, Mueller and Huber2010), which lead solitary lives in the wild, prove themselves adept at learning from social cues.
3. Social learning and asocial learning each come in the same three basic varieties: learning about single stimuli, about relationships among stimuli, and about relationships between stimuli and responses, or actions and outcomes (Heyes Reference Heyes1994; Reference Heyes2011). Each type of social and asocial learning has been found in a wide range of species, including humans (Dawson et al. Reference Dawson, Avarguès-Weber, Chittka and Leadbeater2013; Leadbeater et al. Reference Leadbeater2015).
4. Social learning bears the footprints of associative learning. For example, studies of human decision-making combining mathematical modelling with functional brain imaging have found that the same computations, based on the calculation of prediction error, are involved in processing information from social partners (social learning) and personal experiences of reward (asocial learning) (Behrens et al. Reference Behrens, Hunt, Woolrich and Rushworth2008; Garvert et al. Reference Garvert, Moutoussis, Kurth-Nelson, Behrens and Dolan2015; Hill et al. Reference Hill, Boorman and Fried2016).
The second hypothesis suggests that, in most cases, social learning is selective by virtue of domain-general attentional processing, rather than domain-specific strategic processing. For example, when exposed to two potential models, observers attend more to one model than the other, and therefore learn more from one model than the other; they do not learn equally from both models and then, in a second stage of cognitive processing, decide which of the models they should trust to guide their own behaviour. Evidence consistent with this view comes from studies of selective social learning in children, adults, and nonhuman animals (Heyes Reference Heyes2016a; Reference Heyes2016d; Heyes & Pearce Reference Heyes and Pearce2015). However – and here's the crucial part of my answer to the cognition question – in adults and children older than four or five years, there is evidence that some selective social learning is truly strategic; the observer chooses to trust one model rather than another by applying an explicit, metacognitive rule, such as copy the boat builder with the biggest fleet or copy digital natives (Fleming et al. Reference Fleming, Dolan and Frith2012). In one such study, people used information from another agent – advice about which of two options to choose – to the extent that they believed the advisor to be motivated to help rather than to mislead them (Diaconescu et al. Reference Diaconescu, Mathys, Weber, Daunizeau, Kasper, Lomakina, Fehr and Stephan2014). These beliefs were explicitly stated, and the basic effect – covariation between the advisors’ incentives and the participants’ use of their advice – disappeared when participants were told that the advisors did not know which option they were recommending. Therefore, these results indicate that the participants used an explicitly metacognitive strategy such as copy when the model intends to help.
Thus, my answer to the cognition question is: The selective social learning mechanisms that are specialised for cultural inheritance, that constitute cultural learning, differ from other selective social learning mechanisms in being explicitly metacognitive; they represent who knows in the form of conscious, reportable, domain-specific rules. If this is correct, then research on the development of metacognitive rules showing that they are learned through social interaction (Bahrami et al. Reference Bahrami, Olsen, Bang, Roepstorff, Rees and Frith2012; Güss & Wiley Reference Güss and Wiley2007; Heine et al. Reference Heine, Kitayama, Lehman, Takata, Ide, Leung and Matsumoto2001; Hurks Reference Hurks2012; Li Reference Li2003; Mahmoodi et al. Reference Mahmoodi, Bang, Ahmadabadi and Bahrami2013; Mayer & Träuble Reference Mayer and Träuble2013) provides an answer to the specialisation question; it suggests that the selective social learning mechanisms that constitute cultural learning have been specialised by cultural evolution for cultural evolution. Consistent with this answer, there is a growing body of evidence of cross-cultural variation in the metacognitive social learning strategies used by adults (Efferson et al. Reference Efferson, Richerson, McElreath, Lubell, Edsten, Waring, Paciotti and Baum2007; Eriksson Reference Eriksson2012; Henrich & Broesch Reference Henrich and Broesch2011; Mesoudi et al. Reference Mesoudi, Chang, Murray and Lu2015; Toelch et al. Reference Toelch, Bruce, Newson, Richerson and Reader2014). For example, in contrast with Westerners, Fijians are less likely to seek advice from people with more formal education (Henrich & Broesch Reference Henrich and Broesch2011). Compared to Britons, people from mainland China engage in more social learning, and their social learning is less dependent on uncertainty (Mesoudi et al. Reference Mesoudi, Chang, Murray and Lu2015).
Finally, my answer to the contribution question comes in three steps:
1. Metacognitive social learning strategies are able to focus social learning on knowledgeable agents with greater accuracy and precision because these strategies have been honed by cultural selection.
2. When knowledgeable agents can be identified accurately, individuals and social groups can afford to invest in the development of cognitive mechanisms enabling high-fidelity cultural inheritance of skills.
3. High-fidelity inheritance promotes cultural adaptation by reducing the number of models contributing to each new token of a cultural trait and the degree to which the model's influence is contaminated by asocial learning (Godfrey-Smith Reference Godfrey-Smith2012).
6. Imitation
Imitation is the longest-serving category of cultural learning. Scientists have been claiming for more than a century that imitation involves complex computations specialised by genetic evolution for high-fidelity cultural inheritance, and that this cognitive instinct plays a crucial role in allowing humans to make and use tools (Washburn Reference Washburn1908). Chapter 6 embraces the idea that imitation is special but argues that it is made possible by a culturally inherited mechanism. The selling point of chapter 6 is that it addresses head-on the question of how a new cognitive mechanism could be assembled in the course of ontogeny through social interaction.
Imitation occurs when observation of a model causes the observer to perform topographically similar behaviour, that is, behaviour in which parts of the observer's body move in the same way, relative to one another, as parts of the model's body. Thus, the boy in Figure 5 is imitating the men, not because he is wearing similar clothes and heading in the same direction, but because parts of the boy's body, his arms and torso, are configured – spatially related to one another – in the same way as those of the men. Imitation has been assumed to involve complex, dedicated computations because in many cases, like that in Figure 5, it solves a thorny correspondence problem. When the boy puts his hands behind his back, he doesn't see (or hear or feel) anything resembling what he sees (or hears or feels) when he looks at the men putting their hands behind their backs, and yet somehow the boy's cognitive system has produced an action that looks the same, that corresponds, from a third-party perspective.

Figure 5. An example of imitation.
In the late 1970s, it was reported that newborn human babies can imitate a range of facial expressions and hand movements (Meltzoff & Moore Reference Meltzoff and Moore1977). The reliability and validity of these findings have been questioned repeatedly (Anisfeld Reference Anisfeld1979; Reference Anisfeld, Hurley and Chater2005; Jacobson & Kagan Reference Jacobson and Kagan1979; Jones Reference Jones2006; Reference Jones2007; Reference Jones2009; Koepke et al. Reference Koepke, Hamm, Legerstee and Russell1983; Masters Reference Masters1979; McKenzie & Over Reference McKenzie and Over1983; Meltzoff & Moore Reference Meltzoff and Moore1979). However, replicated and extended in some laboratories, they have led to widespread acceptance of a theory suggesting that the correspondence problem is solved by a black box delivered by the genes. This cognitive instinct theory suggests that humans have an innate device that detects “equivalences between observed and executed acts,” both encoded “supramodally” as “organ relations,” but does not propose computations that would allow organ relations to be derived from observed body movements or cashed out as executed actions (Meltzoff & Moore Reference Meltzoff and Moore1997). Thus, the cognitive instinct theory of imitation says there is a genetically inherited thing that solves the correspondence problem, but it does not say how the thing works. Identifying the thing with mirror neurons (Lepage & Theoret Reference Lepage and Théoret2007) creates another black box. The question “How do people imitate?” becomes the question “How do mirror neurons imitate?”
The alternative, “Associative Sequence Learning” or cognitive gadget, theory of imitation suggests that the correspondence problem is solved by “matching vertical associations” – bidirectional excitatory links between sensory and motor representations of the same action, forged by associative learning during self-observation and specified types of sociocultural interactions (see Fig. 6). This theory offers a mechanistic explanation for the imitation of both familiar actions (sometimes called mimicry) and novel actions (sometimes called true imitation or observational learning). In the latter case, it proposes that, via associative learning, matching vertical associations create a new cognitive mechanism by connecting two domain-general processes that normally operate independently. Matching vertical associations gear perceptual sequence learning, processes that encode the serial order of external stimuli, to motor sequence learning, processes that normally operate only when the agent is learning a new skill, such as riding a bike, through practice (Catmur et al. Reference Catmur, Walsh and Heyes2009; Heyes & Ray Reference Heyes and Ray2000).
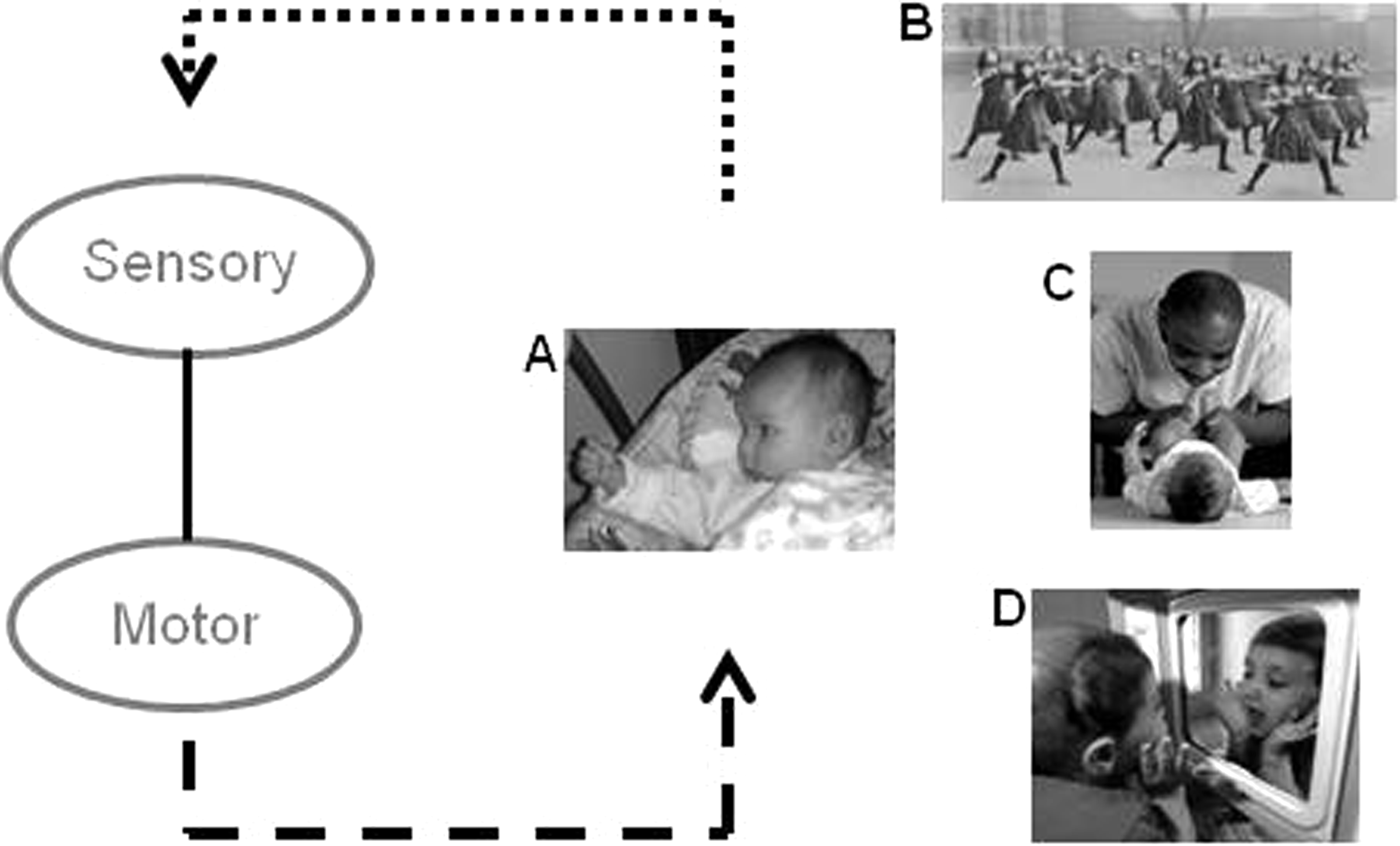
Figure 6. Matching vertical associations are acquired through sensorimotor learning. In the simplest case, self-observation (A), activation of a motor representation contributes to performance of an action (e.g., grasping; dotted arrow), and observation of the performed action produces correlated activation of a corresponding visual representation (dashed arrow). Correlated activation strengthens the excitatory link between the sensory and motor representations, establishing a matching vertical association (solid vertical line). Synchronous activities (B), being imitated by others (C), and optical mirrors (D) provide correlated sensorimotor experience for perceptually opaque actions, such as facial gestures and whole-body movements.
The cognitive instinct theory was recently undermined by a large-scale, longitudinal study of imitation in newborns, which reported negative results for all 11 gestures tested (Oostenbroek et al. Reference Oostenbroek, Suddendorf, Nielsen, Redshaw, Kennedy-Costantini, Davis, Clark and Slaughter2016). In contrast, the gadget theory is supported by evidence of two kinds (for reviews, see Catmur et al. Reference Catmur, Walsh and Heyes2009; Reference Catmur, Press, Heyes, Murphy and Honey2016; Cook et al. Reference Cook, Bird, Catmur, Press and Heyes2014): Training studies involving adults, infants, and nonhuman animals show that imitation – measured behaviourally and via mirror responses in the brain – can be enhanced, abolished, and reversed by novel sensorimotor experience. For example, adults usually do not imitate the actions of inanimate systems, such as robots, but after a brief period of training in which robotic movements are paired with topographically similar body movements performed by the observer, people imitate robots as much as they imitate other people (Press et al. Reference Press, Bird, Flach and Heyes2005). The second kind of evidence indicates that imitation, although flexible and adaptive, has the signature limits (Butterfill & Apperly Reference Butterfill and Apperly2013) one would expect if it is controlled by matching vertical associations. For example, imitation learning is effector-dependent; it does not readily generalise across parts of the body. People who have observed a complex sequence of key-pressing movements can reproduce the sequence when their fingers are in the same keyboard positions as the fingers of the model, but they cannot imitate the sequence when their hands are crossed on the keyboard (right hand operates left keys, and vice versa), or when they are asked to use their thumbs rather than their fingers to press the keys (Bird & Heyes Reference Bird and Heyes2005; Leighton & Heyes Reference Leighton and Heyes2010).
The final section of chapter 6 addresses five objections to the cognitive gadget theory of imitation, emphasising the following points.
1. Intervention versus development. Like most scientific evidence, the results of training studies – and related studies of expertise (e.g., Calvo-Merino et al. Reference Calvo-Merino, Grèzes, Glaser, Passingham and Haggard2006) – support inference to the best explanation, not deduction (Lipton Reference Lipton2003). They favour the gadget theory over the instinct theory because they are exactly what the gadget theory predicts but difficult for the instinct theory to accommodate.
2. Homo imitans. Humans are more skilled and prodigious imitators than other animals, not primarily because they have better resources “on the inside” (e.g., higher capacity mechanisms of associative learning), but because they have superior resources “on the outside,” cultural artefacts and practices that support the acquisition of matching vertical associations.
3. Intentionality. A matching vertical association for an action, x, makes it possible, not obligatory, to imitate x.
4. Overimitation. Children's propensity to imitate instrumentally superfluous features of action (Lyons et al. Reference Lyons, Young and Keil2007) raises questions about the motivation, rather than ability, to imitate. Although the gadget theory is concerned with ability rather than motivation, it is consistent with evidence that overimitation is due to reinforcement learning (Baer & Sherman Reference Baer and Sherman1964; Garcia et al. Reference Garcia, Baer and Firestone1971; Grusec & Abramovitch Reference Grusec and Abramovitch1982; Young et al. Reference Young, Krantz, McClannahan and Poulson1994).
5. What's the use? The gadget model raises the possibility that evolutionists have overlooked the most important function of imitation: high-fidelity cultural inheritance, not of object-directed actions, but of communicative and gestural skills (Heyes Reference Heyes, Sterelny, Joyce, Calcott and Fraser2013).
7. Mindreading
Mindreading, the ascription of mental states, is classed as a form of cultural learning because it is likely to be the special ingredient of human teaching. Effective teaching involves many other cognitive and motivational ingredients, including social tolerance and attentiveness, but mindreading stands out as the most likely candidate for a human-specific cognitive adaptation for teaching.
The idea that mindreading is a genetic adaptation, a cognitive instinct, begins to be less compelling when one compares mindreading with print reading (literacy), a distinctively human cognitive mechanism that is known to be a product of cultural evolution (Heyes & Frith Reference Heyes and Frith2014; see Sect. 1). For example, studies of neural specialization (Van Overwalle Reference Van Overwalle2009), cultural variation (Shahaeian et al. Reference Shahaeian, Peterson, Slaughter and Wellman2011), and genetically heritable developmental disorders (autism; Frith Reference Frith2001), have all been treated as evidence that mindreading is a cognitive instinct, and yet print reading shows comparable degrees of neural specialisation (Dehaene & Cohen Reference Dehaene and Cohen2011) and cultural variation (Changizi et al. Reference Changizi, Zhang, Ye and Shimojo2006), and is associated with genetically heritable developmental disorders of its own (dyslexias; Paracchini et al. Reference Paracchini, Scerri and Monaco2007).
At 5 years of age, monozygotic twins are no more alike than dizygotic twins in their mindreading ability (Hughes et al. Reference Hughes, Jaffee, Happé, Taylor, Caspi and Moffitt2005). This suggests negligible genetic influence and a powerful role for learning in the development of individual differences in mindreading, but it does not tell us what kind of learning is important. In principle, it could be the kind of introspection-based learning emphasised by simulation theory; the science-like learning postulated by theory-theory, in which the child tests her self-generated hypotheses against a database of observed behaviour; or, as gadget theory suggests, a form of cultural inheritance in which mindreading experts – parents and others – instruct children about the mind, in conversation and by structuring developmental environments. In chapter 7 of Cognitive Gadgets, I argue that evidence from natural experiments (Mayer & Träuble Reference Mayer and Träuble2013; Pyers & Senghas Reference Pyers and Senghas2009), observational studies (Meins Reference Meins, Siegal and Surian2012; Meristo et al. Reference Meristo, Hjelmquist and Morgan2012; O'Brien et al. Reference O'Brien, Slaughter and Peterson2011; Slaughter & Peterson Reference Slaughter, Peterson, Siegal and Surian2012; Taumoepeau & Ruffman Reference Taumoepeau and Ruffman2006; Reference Taumoepeau and Ruffman2008), and traditional experiments (de Villiers & de Villiers Reference De Villiers and de Villiers2012; Lohmann & Tomasello Reference Lohmann and Tomasello2003) favours the third of their possibilities. For example, in a natural experiment, deaf people who had been deprived of conversation about the mind because they learned Nicaraguan Sign Language (NSL) when it included very few mental-state terms were less likely to pass a false-belief test than a second cohort who had learned NSL later, when it contained a wider range of mental-state terms (Pyers & Senghas Reference Pyers and Senghas2009). The first cohort was 10 years older than the second cohort; they had had 10 more years in which to introspect and test hypotheses. Therefore, if introspection or science-like learning, rather than conversation, were crucial for the development of mindreading, one would expect the first cohort to be better, not worse, at ascribing false belief.
Studies of implicit mindreading using eye-movement indices of behaviour prediction imply that nonhuman apes (Krupenye et al. Reference Krupenye, Kano, Hirata, Call and Tomasello2016) and prelinguistic infants (Kovács et al. Reference Kovács, Téglás and Endress2010; Onishi & Baillargeon Reference Onishi and Baillargeon2005) are capable of ascribing false beliefs. According to the continuity interpretation, implicit mindreading is mediated by the same, specialized cognitive mechanisms that mediate explicit mindreading in deliberating adults (Baillargeon et al. Reference Baillargeon, Scott and He2010). If the continuity interpretation is correct, mindreading could not be a cognitive gadget because it develops without (apes) and before (infants) conversation about the mind. Two other interpretations of implicit mindreading are compatible with the cognitive gadget theory, however. According to the “two-systems” (Apperly Reference Apperly2010; Perner Reference Perner, Frensch and Schwarzer2010) and “submentalizing” (Heyes Reference Heyes2014a; Reference Heyes2014b; Reference Heyes2015; Reference Heyes2017b) interpretations, implicit and explicit mindreading depend on different cognitive mechanisms. The two-systems view proposes that the mechanisms mediating implicit mindreading are specialised for fast and efficient representation of mental states, while the submentalizing view suggests that they are domain-general mechanisms, representing relatively low-level features of action stimuli – such as colour, shape, and movement – rather than mental states. Evidence that concurrent demands on executive function interfere with explicit mindreading (Bull et al. Reference Bull, Phillips and Conway2008), but not with implicit mindreading (Qureshi et al. Reference Qureshi, Apperly and Samson2010), and that people with autism can engage in explicit mindreading despite impairments in implicit mindreading favour the two-systems and submentalizing hypotheses over the continuity hypothesis (Senju et al. Reference Senju, Southgate, White and Frith2009).
The cognitive gadget theory implies that children learn to read minds through language and therefore appears to be in direct opposition to the Gricean view that ascription of mental states is a precondition for linguistic communication (Bloom Reference Bloom2000; Sperber & Wilson Reference Sperber and Wilson1995; Tomasello Reference Tomasello1999). For two reasons, however, I suspect that the cognitive gadget theory and the Gricean view of language are reconcilable. First, Grice offered a rational reconstruction, rather than a psychologically realistic account, of what is happening when people talk to one another (Sperber Reference Sperber and Aunger2000). Second, Moore (Reference Moore2016; Reference Moore2017) has argued persuasively that Gricean communication can get off the ground – in evolutionary and developmental time – with minimal mindreading; all that is needed is “a basic understanding of others’ purposive activities and desires [which I would characterise as knowledge of action-outcome relationships], operating in conjunction with some tracking what others had or had not seen [or viewed]” (p. 19).
Thus, advancing an alternative to simulation theory and theory-theory, chapter 7 argues that mindreading is culturally inherited – a cognitive gadget. Expert mindreaders communicate mental state concepts, and ways of representing those concepts, to novices. As the present generation of novices become expert, they pass on the knowledge and skill of mindreading to the next cultural generation.
8. Language
I have been thinking about social learning, imitation, and mindreading for a long time, but I write about language as an outsider. While developing the ideas in Cognitive Gadgets, I immersed myself for the first time in research on the origins of language, expecting to find clear evidence that language is a cognitive instinct – one on which gadgets are built. Instead, I found a wealth of evidence that language is itself a gadget and a divide between genetic and cultural evolutionists that no longer appears to be resolvable by empirical means.
Chapter 8 begins by contrasting a gradualist genetic theory of language evolution (Culicover & Jackendoff Reference Culicover and Jackendoff2005; Pinker Reference Pinker1994; Pinker & Bloom Reference Pinker and Bloom1990) with a cultural theory of language evolution (Christiansen & Chater Reference Christiansen and Chater2016), and then discusses evidence that should, or is widely thought to, support one of these theories over the other. The evidence relates to linguistic universals, critical periods, neural localisation, domain-general sequence learning, and social shaping.
8.1. Linguistic universals
There are few, if any, non-definitional features that all languages have in common (Evans & Levinson Reference Evans and Levinson2009; Everett Reference Everett2005; Jelinek Reference Jelinek, Bach, Jelinek, Kratzer and Partee1995). However, this is compatible with the genetic theory when linguistic universals are construed not as features that all or many languages have in common, but as components of Universal Grammar, or a genetically inherited language of thought (Berwick & Chomsky Reference Berwick and Chomsky2015). A “universal” in this sense need not be present in all or even most natural languages, and a feature that was found to be present in all languages would not necessarily be a universal (Boeckx Reference Boeckx2006; Chomsky Reference Chomsky1965; Pinker & Jackendoff Reference Pinker and Jackendoff2005)
8.2 Critical periods
Research with migrant populations and native speakers indicates that second-language proficiency depends on number of years of exposure to the second language, rather than on whether learning began before or after puberty (Birdsong & Molis Reference Birdsong and Molis2001; Flege et al. Reference Flege, Yeni-Komshian and Liu1999; Hakuta et al. Reference Hakuta, Bialystok and Wiley2003), and that, with the exception of phonology (Werker & Hensch Reference Werker and Hensch2015), first- and second-language learners may obtain similar levels of proficiency (Dabrowska Reference Dąbrowska2012). These findings suggest that, contrary to the claims of some genetic theorists (Lenneberg Reference Lenneberg1967; Pinker Reference Pinker1994), grammar learning is not a critical-period phenomenon; however, the critical-period claim is not an original or essential part of the genetic account of the evolution of language.
8.3 Neural localisation
Language enlists a more widely distributed set of brain areas than any other major psychological function (Anderson Reference Anderson2008), and Broca's area is more often active during non-linguistic than linguistic tasks (Poldrack Reference Poldrack2006). These data certainly tell against the idea that there is a “language centre,” but it is not clear why it was ever supposed that genetically inherited information is more likely than culturally inherited information to be found in a narrowly localised area of the brain (Cowie Reference Cowie and Zalta2016; Lenneberg Reference Lenneberg1967; Pinker Reference Pinker1994).
8.4. Domain-general sequence learning
Computer simulation indicates that sequence learning, without inbuilt language-specific constraints, enables a system to process complex grammatical constructions in a human-like way (Christiansen & MacDonald Reference Christiansen and MacDonald2009). Experiments examining individual differences in typically developing adults and children suggest that they use the same sequence learning processes to learn artificial and “real,” linguistic grammars (Kidd Reference Kidd2012; Kidd & Arciuli Reference Kidd and Arciuli2016; Misyak & Christiansen Reference Misyak and Christiansen2012). Studies of people with specific language impairment indicate that their impairment is not, in fact, specific to language (Hsu & Bishop Reference Hsu and Bishop2014; Hsu et al. Reference Hsu, Tomblin and Christiansen2014; Tomblin et al. Reference Tomblin, Mainela-Arnold and Zhang2007). Likewise, research with nonhuman animals confirms that domain-general sequence learning capacity has increased in the hominin line (Wilson et al. Reference Wilson, Slater, Kikuchi, Milne, Marslen-Wilson, Smith and Petkov2013) and provides a plausible model of how this change has been implemented in the primate brain (Bornkessel-Schlesewsky et al. Reference Bornkessel-Schlesewsky, Schlesewsky, Small and Rauschecker2015; Ivanova et al. Reference Ivanova, Isaev, Dragoy, Akinina, Petrushevskiy, Fedina, Shklovsky and Dronkers2016). It also supports evidence from humans that mutations of FOXP2 interfere with language by interfering with sequence learning more generally and that FOXP2 is not a “language gene” (Reimers-Kipping et al. Reference Reimers-Kipping, Hevers, Pääbo and Enard2011; Schreiweis et al. Reference Schreiweis, Bornschein, Burguière, Kerimoglu, Schreiter, Dannemann, Goyal, Rea, French, Puliyadi and Groszer2014).
8.5. Social shaping
Research on social shaping suggests that infants and children are frequently corrected by adults when they make grammatical errors (Bohannon et al. Reference Bohannon, MacWhinney and Snow1990; Demetras et al. Reference Demetras, Post and Snow1986; Moerk Reference Moerk1991), and that this negative input is put to use in language learning (Street & Dabrowska Reference Street and Dąbrowska2010; Taumoepeau Reference Taumoepeau2016). These findings, like those on sequence learning, confirm novel predictions of the cultural theory, and, in the case of social shaping, challenge Chomsky's “poverty of the stimulus argument,” a foundation of the genetic account.
The genetic theory is proving remarkably resilient in the face of what appear to be empirical defeats (linguistic universals, critical periods, neural specialisation), and a tide of positive evidence supporting the cultural theory (sequence learning, social shaping). Some of this resilience may be due to the motility of the genetic theory. Chomsky's view has changed radically since the 1950s, but each of his successive approaches is represented in the current literature (Boeckx Reference Boeckx2006; Crain et al. Reference Crain, Goro and Thornton2006; Culicover & Jackendoff Reference Culicover and Jackendoff2005; Pinker & Jackendoff Reference Pinker and Jackendoff2005). The genetic theory is also insulated by the competence-performance distinction (Chomsky Reference Chomsky1965). This enables its proponents to argue that, for example, research on sequence learning and social shaping bears on the externalisation of language (performance), but not on whether there is a genetically inherited language of thought (competence). Some of the resilience may even come from historically deep convictions about the significance of language; the genetic theory more fully preserves the idea that language is a Rubicon separating humanity from the beasts. As an outsider, I can only conclude that, although the genetic theory of language evolution is appealing for a variety of reasons (some of them extra-scientific), the cultural theory – once a poor relation – is now clearly specified and rich in empirical support.
9. Cultural evolutionary psychology
The final chapter of Cognitive Gadgets returns to some of the evolutionary questions in chapter 2, now with concrete examples from the case studies, and discusses the prospects for a cultural evolutionary psychology.
9.1. Cultural group selection
Attempting to make the cognitive gadgets hypothesis as clear as possible, I try to spell out who benefits from the cultural selection of cognitive mechanisms and the nature of the benefit. This analysis allows two types of multilevel selection (CGS 1 and CGS 2; Damuth & Heisler Reference Damuth and Heisler1988; Okasha Reference Okasha2005) and uses imitation as an example (see Fig. 7): Imagine a human population divided into two social groups, X and Y, defined geographically or culturally, not by genes. Each person has an imitation mechanism, gearing motor sequence learning to perceptual sequence learning via matching vertical associations (Ch. 6). There are two versions of this mechanism: M and M′; M′ is less common in X than Y. The M′ version has a richer repertoire of matching vertical associations for whole-body movements than the M version, enabling people with M′ more accurately to imitate actions involved in ritual (e.g., dance), hunting (e.g., stalking), and combat (e.g., spear throwing). As a consequence, bearers of M′ are better able than bearers of M to cooperate in a range of tasks (Heyes Reference Heyes, Sterelny, Joyce, Calcott and Fraser2013; Tarr et al. Reference Tarr, Launay, Cohen and Dunbar2015; Tunçgenç & Cohen Reference Tunçgenç and Cohen2016), and to sustain the cultural inheritance of techniques that enhance success in hunting and intergroup combat. These advantages lead groups in which the M′ mechanism predominates to acquire greater numbers of new members (CGS 1 in Fig. 7), or to produce more descendent groups (CGS 2 in Fig. 7), than groups in which M predominates.

Figure 7. Two types of fitness in cultural group selection.
9.2. Inheritance
The cultural inheritance of cognitive mechanisms involves social processes such as conversation, storytelling, turn-taking, collective reminiscing, teaching, demonstrating, and engaging in synchronous drills. For example, through conversation, teaching, and demonstration, children learn to deploy metacognitive social learning strategies in the same way as the people around them (Ch. 5). Through taking turns in face-to-face interaction and engaging in synchronous drills, children acquire a particular repertoire of matching vertical associations; they become able to imitate the same range of actions as their cultural parents (Ch. 6). Through conversation, storytelling, and collective reminiscing, children become able to represent mental states and accumulate a stock of generalisations about the way mental states relate to one another, to behaviour, and to the world (Ch. 7; Nile & Van Bergen Reference Nile and Van Bergen2015; Salmon & Reese Reference Salmon and Reese2016). Dedicated research of a radically new kind is needed to measure the robustness of these inheritance mechanisms. In advance of such research, three considerations suggest that they are robust enough to support cultural group selection of cognitive processes:
1. High-fidelity replication is not a requirement for Darwinian selection (Godfrey-Smith Reference Godfrey-Smith2012).
2. Redundancy is built into distributed inheritance (e.g., mindreading via vertical, horizontal and oblique routes).
3. Each social process of inheritance occurs repetitively, delivering multiple learning trials. Children are told a particular story not once but many times. Different stories contain the same themes, morals, and tropes. Adults imitate the same facial gestures over and over again in face-to-face interaction with infants. Collective reminiscence returns repeatedly to the same episodes.
9.3. Genetic assimilation
In principle, it is possible that new cognitive mechanisms start out as cognitive gadgets, constructed in the course of development through social interaction, but then selection progressively favours genetic mutations that reduce the experience-dependence of the gadgets’ development, converting them into cognitive instincts (Henrich Reference Henrich2015). In practice, I have looked for, and failed to find, empirical evidence that this kind of genetic assimilation has occurred – for example, evidence that learning is faster in natural than unnatural conditions. Cognitive gadgets may resist genetic assimilation because distinctively human cognitive mechanisms need to be nimble. Their job is to track specific, labile features of the environment, which move too fast for genetic evolution. For example, social learning strategies track “who knows” in a particular social group, something that changes with shifting patterns in the division of labour and therefore of expertise. Imitation tracks communicative gestures, ritual movements, and manual skills that change as groups find new group markers, bonding rituals, and technologies. And mindreading, like language, must track not only externally driven change in the phenomena it seeks to describe, but also self-generated change: alterations in the way the mind works caused by shifts in the regulative properties of theory of mind (McGeer Reference McGeer, Hutto and Ratcliffe2007).
9.4. A little history
The cognitive gadgets hypothesis is a force theory rather than an historical theory; it is concerned with the processes involved, rather than the history of events, in human evolution. The ideal theory would be high on both the force and historical dimensions. Therefore, connecting the cognitive gadgets theory to key events in human evolution, using the archaeological record, is a priority for future research. Making a start down that road and building on the “collective intelligence” hypothesis (Henrich Reference Henrich2004b; Reference Henrich2015; Kline & Boyd Reference Kline and Boyd2010; Muthukrishna & Henrich Reference Muthukrishna and Henrich2016; Richerson & Boyd Reference Richerson, Boyd, Hatfield and Pittman2013; Sterelny Reference Sterelny, Newen, de Bruin and Gallagher2018), I suggest that climate-driven demographic changes around 250,000 years ago launched not only the cultural evolution of knowledge and skills, but also the cultural evolution of distinctively human cognitive mechanisms. The Small Ordinary components of the genetic starter kit were already in place (chap. 3) and had been supporting cooperation and simple stone technologies for millions of years. Demographic changes allowed the Small Ordinary components to begin to be elaborated by cultural group selection into the Big Special mechanisms that we now identify as, for example, causal understanding, episodic memory, imitation, theory of mind, and full-blown language.
9.5. Human nature
Cultural evolutionary psychology is consistent with an evolutionary causal essentialist conception of human nature: a hybrid of the nomological account (Machery Reference Machery2008; Reference Machery, Hannon and Lewens2018) and causal essentialist theories (Samuels Reference Samuels2012). On this view, human nature is the set of mechanisms that underlie the manifestation of species-typical cognitive and behavioural regularities that humans tend to possess as a result of the evolution of their species; crucially, evolution encompasses all selection-based evolutionary processes – genetic, epigenetic, and cultural. The primary implication of evolutionary causal essentialism is that human nature is labile; it changes over historical rather than geological time. The first signs of literacy date from about 6,000 years ago, and now, the cognitive gadgets that enable people to read, being present in more than 80%–90% of the global population, are part of human nature. On a broader scale, cultural evolutionary psychology implies that human minds are more agile, but also more fragile, than previously thought. We are not stuck in the Pleistocene past with Stone Age minds, and new technologies – social media, robotics, virtual reality – provide the stimulus for further cultural evolution of the human mind. However, we have more to lose. Wars and epidemics can wipe out not just know-how, but also the means to acquire that know-how. The capacity for cultural evolution, as well as the products of cultural evolution, could be lost.
9.6. Cultural evolutionary psychology
The idea at the core of Cognitive Gadgets – that cultural evolution shapes distinctively human cognitive mechanisms – is a bold, testable hypothesis. Of the mechanisms examined in the case studies, selective social learning provides the freshest opportunity for research by cognitive scientists. Beyond the case studies, there are many other mechanisms to be explored, including causal understanding and episodic memory. Moral reasoning is a priority because it is a form of cultural learning, and, being so intimately connected with emotion, has the potential to cast light on the co-evolution of cognitive and emotional gadgets (Barrett Reference Campbell2017).
One of the strengths of cultural evolutionary psychology is that it brings into sharp focus questions about how a new cognitive mechanism is put together over time, and makes them tractable. Evolutionary psychologists tend to assume that, if something is a cognitive instinct, it is the responsibility of some other discipline (perhaps genetics or paleo-archaeology), not cognitive science, to explain how it was constructed (Samuels Reference Samuels2004). In contrast, cultural evolutionary psychology encourages cognitive scientists and others to develop and test theories about gadget construction. Furthermore, because cultural evolution is faster than genetic evolution, and much of the construction process occurs within lifetimes, the cognitive gadgets theory makes questions about construction empirically tractable. They can be addressed, in collaboration with anthropologists and historians, by research involving contemporary and historical populations, as well as those for which we have only archaeological evidence. We don't have to guess how cognitive mechanisms were put together by genetic evolution in the Pleistocene past; through laboratory experiments and field studies, we can watch them being built in people alive today.
Cognitive Gadgets opens up a third way. It suggests that distinctively human cognitive mechanisms are adaptive because they are shaped primarily not by nature or nurture but by culture. I tried in the book to make this hypothesis clear and plausible, but I have no illusions that the case is already conclusive. A great deal more work is needed to test the cognitive gadgets theory, and, through the lens of cultural evolutionary psychology, to develop a deeper understanding of the origins and operating characteristics of human minds.












Target article
Précis of Cognitive Gadgets: The Cultural Evolution of Thinking
Related commentaries (17)
Cognitive gadgets and cognitive priors
Cognitive gadgets and genetic accommodation
Cognitive gadgets: A provocative but flawed manifesto
Could nonhuman great apes also have cultural evolutionary psychology?
Cultural evolutionary psychology is still evolutionary psychology
Culture in the world shapes culture in the head (and vice versa)
Executive functions are cognitive gadgets
How is mindreading really like reading?
Imitation: Neither instinct nor gadget, but a cultural starting point?
Instincts or gadgets? Not the debate we should be having
Keeping cultural in cultural evolutionary psychology: Culture shapes indigenous psychologies in specific ecologies
Language is not a gadget
Mending wall
Mills made of grist, and other interesting ideas in need of clarification
Sociocultural memory development research drives new directions in gadgetry science
Tinkering with cognitive gadgets: Cultural evolutionary psychology meets active inference
Twenty questions about cultural cognitive gadgets
Author response
Cognition blindness and cognitive gadgets