Helicobacter pylori is a Gram-negative, spiral-shaped micro-organism which has morphological characteristics penetrate the mucosa and colonise the stomach and duodenum(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1,Reference Lopes, Nunes and Martins2) . H. pylori infection is one of the most common chronic bacterial infections, affecting approximately 4·4 billion individuals worldwide(Reference Hooi, Lai and Ng3). Reports of infection prevalence rates widely range among geographic regions, reaching the highest levels in developing countries(Reference Savoldi, Carrara and Graham4). H. pylori infection causes chronic progressive gastric inflammation and a variety of diseases, including gastric and duodenal ulcers and gastric cancer(Reference Malfertheiner, Megraud and O’Morain5). The WHO has classified H. pylori as a group I carcinogen with a risk of stomach cancer(Reference Mbulaiteye, Hisada and El-Omar6,Reference Testerman and Morris7) . Generally, H. pylori infection increases the risk of malignancy and the expense of H. pylori-associated morbidity(Reference Testerman and Morris7,Reference Kim, Choi and Chung8) . Eradication of H. pylori infection has been proven to reduce the incidence of gastric cancer(Reference Chiang, Chang and Chen9,Reference Holmes, Rios and Berice10) .
Selection of the best drug regimens for eradication of H. pylori infection is already challenging(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1). The treatment plan currently adopted as a first-line option includes a combination of a proton pump inhibitor, clarithromycin and amoxicillin or metronidazole/tinidazole, according to international guidelines(Reference Zullo, Hassan and Ridola11,Reference Malfertheiner, Megraud and O’Morain12) . This therapy persists during 7–14 d, twice a day(Reference Zullo, Hassan and Ridola11). Eradication rates of H. pylori treated with a 14-d triple therapy reached only 70 % in non-ulcer dyspepsia patients and 80 % in patients with peptic ulcers(Reference Zullo, Hassan and Ridola11). The success rate in most European, Asian and North American countries is constantly declining, and low cure rates with 20–45 % have been recently reported(Reference Selgrad and Malfertheiner13).
This eradication rate is distant from the desirable rate of infectious diseases and from that proposed by the WHO(Reference Malfertheiner, Megraud and O’Morain12,Reference Bardonnet, Faivre and Boullanger14) . The main limitation of the current therapy results from the lack of therapeutic compliance, due to the incidence of side effects and the discomfort resulting from the multiple doses(Reference Patel and Patel15,Reference Roszczenko-Jasińska, Wojtyś and Jagusztyn-Krynicka16) . These factors may lead to the development of antibiotic resistance(Reference Roszczenko-Jasińska, Wojtyś and Jagusztyn-Krynicka16). Moreover, antimicrobial agents such as amoxicillin and clarithromycin are degraded by gastric acid(Reference Bardonnet, Faivre and Boullanger14). Therefore, it is necessary to use higher doses which are reflected in the increase of gastrointestinal side effects, and consequently the discontinuation of the therapy(Reference Roszczenko-Jasińska, Wojtyś and Jagusztyn-Krynicka16). The bacteria are sensitive to other antimicrobial drugs, nevertheless they cannot be used in the acidic medium(Reference Bardonnet, Faivre and Boullanger14). Despite all the endeavours, the current therapy presents many limitations which have led to the failure of H. pylori eradication(Reference Lopes, Nunes and Martins2). To overcome these limitations, modification of therapeutic strategies(Reference Malfertheiner, Megraud and O’Morain5) and novel effective therapies have been proposed, including phytomedicine(Reference Vítor and Vale17). So far, many compounds found in dietary and medicinal plants, herbs and fruit extracts have been shown to possess antimicrobial activities(Reference Grozdanova, Trusheva and Alipieva18).
Cranberry (Vaccinium macrocarpon), belonging to the Ericaceae family, is a fruit widely consumed in many countries(Reference Skrovankova, Sumczynski and Mlcek19). Cranberry contains a number of phytochemicals which have bioactive properties when consumed, including proanthocyanidins, anthocyanin pigments, flavonol glycosides and certain acids(Reference Howell20). Also, cranberries have been highly ranked in terms of their antioxidant capacity and are known as a rich source of phenolic compounds.
In recent studies, cranberry has been tested both in vitro and in clinical trials and promised a non-pharmacological treatment to manage H. pylori infections(Reference Howell20). In vitro studies demonstrated that cranberry extract especially containing A-type proanthocyanidins prevented adhesion of H. pylori sialic acid-specific strains to human gastric mucus and stomach cells(Reference Li, Ma and Guo21). Also, cranberry has been clinically shown to suppress infections when regularly consumed(Reference Li, Ma and Guo21). Some studies have shown that cranberry juice constituents inhibited the adhesion of a wide range of microbial pathogens, including H. pylori, E. coli and oral bacteria (1) while others did not find any significant effect(Reference Li, Ma and Guo21,Reference Shmuely, Yahav and Samra22) . Surprisingly, in a double-blind randomised, placebo-controlled trial (RCT) of H. pylori positive individuals, there was no statistically significant difference in eradication rates across comparison groups(Reference Shmuely, Yahav and Samra22).
However, results from human studies have remained inconclusive. Overall, given the presence of conflicting results on the effect of cranberry extract on H. pylori eradication, to summarise the evidence and clarify these inconsistencies in the results of human trials, the current systematic review and meta-analysis were conducted to systematically identify and quantitatively assess the efficacy of cranberry supplementation on H. pylori-positive individuals.
Methods
Protocol registration
The protocol has been registered on the PROSPERO website as CRD42021232808, available from: https://www.crd.york.ac.UK/prospero/display_record.php?ID=CRD42021232808.
Search strategy
A comprehensive literature search was carried out to identify and appraise investigations which had assessed the effects of cranberry supplementation on H. pylori eradication. Electronic databases, including Pubmed (http://www.ncbi.nlm.nih.gov/pubmed), Scopus (http://www.scopus.com), Cochrane Library (http://www.cochranelibrary.com), Web of Knowledge (http://www.webofscience.com) and Google Scholar (http://scholar.google.com) were browsed up to 26 December 2020.
The following keywords in any possible combination were used in the Pubmed search strategy: ((Cranberry (tiab) OR Vaccinium macrocarpon (tiab) OR Viburnum (tiab) OR Cranberry supplementation (tiab) OR Vaccinium (tiab) OR Vaccinium oxycoccus (tiab) OR Vaccinium erythrocarpum (tiab) OR Cranberry juice (tiab) OR Cranberry extract) AND (Helicobacter pylori (tiab) OR H. pylori (tiab) OR Helicobacter pylori Eradication (tiab) OR H. pylori Eradication)).
The following keywords were used in the Scopus search strategy: ((TITLE-ABS-KEY Cranberry TITLE-ABS-KEY OR Vaccinium macrocarpon TITLE-ABS-KEY OR Viburnum TITLE-ABS-KEY OR Cranberry supplementation TITLE-ABS-KEY OR Vaccinium TITLE-ABS-KEY OR Vaccinium oxycoccus TITLE-ABS-KEY OR Vaccinium erythrocarpum TITLE-ABS-KEY OR Cranberry juice TITLE-ABS-KEY OR Cranberry extract) AND (Helicobacter pylori TITLE-ABS-KEY OR H. pylori TITLE-ABS-KEY OR Helicobacter pylori Eradication TITLE-ABS-KEY OR H. pylori Eradication)).
The search was independently carried out by two authors (RN and ZR), and the obtained articles were assessed. Also for screening, they assessed the articles through the review of titles and abstracts; and if necessary, full texts. After screening, the full texts of remained articles were evaluated based on inclusion criteria to identify the studies eligible for this systematic review and meta-analysis. At each stage, doubtful cases were discussed within the research team.
The references from selected articles and reviews were manually searched to identify references which may have been missed in our primary search and additional studies. No language, population or publication year restrictions were enforced. All searched studies were included in the Endnote software for screening.
Trial registers have been searched using Current Controlled Trials (http://www.controlled-trials.com/), Clinical-Trials.gov (http://clinicalTrials.gov/ct2/home) and the WHO International Trials Registry Platform search portal (http://www.who.int/trialsearch/ Default.aspx). Iranian Registry of Clinical Trials (https://www.irct.ir/) and the Grey literature have been searched using Open Grey (http://www.opengrey.eu).
Study selection
The following pre-specified inclusion criteria were used:
(1) parallel or crossover RCT; (2) RCT with patients were defined as infected by H. pylori if they tested positive by any of the following tests: the histology, culture, serology, stool antigen, urea breath test or rapid urease test; (3) comparison of the intervention group consumed cranberry juice/extract/powder v. placebo or non-placebo control for suppression of H. pylori; (4) RCT with at least 1 week duration of intervention; (5) RCT which reported H. pylori eradication or suppression as the outcome and clinical trials with an additional intervention group were considered as two separate studies and (6) enrolled adult participants (aged > 18 years).
The following was defined as exclusion criteria:
(1) duplicated data; (2) those with a cohort, cross-sectional and case–control design, review articles and ecological studies.
Screening and final selection of articles were performed based on the inclusion and exclusion criteria by two authors (RN and ZR) separately and independently. In the event of a dispute, the views of the third author (YM) were applied to resolve it.
Data extraction
Two independent investigators (RN and ZR) performed data extraction from each eligible RCT. The following information was extracted:
(1) the first author’s last name; (2) the year of publication; (3) the study location (the country); (4) the study design; (5) individuals’ characteristics (the mean age and sex); (6) the sample size (control and intervention groups); (7) the type of cranberry prescribed; (8) the dosage of cranberry; (9) the duration of intervention; (10) the type of intervention in comparison groups; (11) participant health conditions; (12) the outcome assessment method throughout the trial for the intervention and control groups and (13) the main outcome.
Quality assessment checklists
The Cochrane Risk of Bias Tool for Randomised Controlled Trials(Reference Moher, Shamseer and Clarke23) was used by two separate authors (RN and ZR) to explore potential risks of bias. These scales include items to assess the adequacy of random sequence generation, allocation concealment, blinding as well as the detection of incomplete outcome data, selective outcome reporting and other potential sources of bias. Based on the recommendations of the Cochrane Handbook, judgement of each item was recorded as the ‘Low’, ‘High’ or ‘Unclear’ risk of bias.
A ‘high risk’ score was given to each domain if the study comprised methodological defects which may have affected its findings, a ‘low risk’ score if there was no defect for that domain and an ‘unclear risk’ score if the information was not sufficient to determine the impact (Fig. 2).
Any disagreement in the data extraction and the risk of bias assessment was settled by a third researcher (YM).
Also, this study was performed in accordance with the Jadad score checklist for the Quality Assessment Checklist for Individual Studies for Systematic Reviews and Meta-Analyses and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses instructions(Reference Moher, Shamseer and Clarke23).
Statistical analysis
The ultimate goal of this study was to determine whether the cranberry group had a higher H. pylori eradication rate than the placebo group. OR were used to measure the effect of cranberry supplementation on H. pylori eradication rates using the random effects model. Heterogeneity in meta-analysis may be defined as ‘the variability between studies in the estimates of effects’ and can be categorised as statistical heterogeneity, methodological heterogeneity and clinical heterogeneity. The potential sources of clinical heterogeneity include different study participants, different interventions and different outcome measures across individual studies. Statistical heterogeneity was analysed with χ 2 distribution, Cochran’s Q-test and I 2 statistics. Inter-study heterogeneity was quantitatively explored using Cochran’s Q and I 2 statistics. In this regard, I 2 values of 50 and 75 % indicated substantial and considerable heterogeneity, respectively(Reference Higgins and Thomas24). The I 2 statistic was under 50 % and/or the Q-test was not significant at P < 0·05.
Based on the detected heterogeneity between studies, a random effects or fixed model was applied in the meta-analysis(Reference Higgins and Thomas24,Reference Moher, Jadad and Nichol25) . To obtain the overall effect sizes, we applied a random effects model which took between-studies variations into account. Effect sizes were presented as the OR with 95 % CI, and P-values < 0·05 were considered statistically significant.
In order to assess potential sources of heterogeneity in the meta-analysis, the association between the treatment effect and other study characteristics is often used to methods such as meta-regression and subgroup analysis, including the duration of the intervention (≥ 50 v. < 50 d) and (≥ 100 v. < 100 d) and the Jadad score. We drew a funnel plot to evaluate the publication bias.
Sensitivity analysis was used to detect the dependency of the overall effect size on a particular study. In addition, the Egger test was used to assess publication bias. All analyses were carried out through the application of the metan and metabias commands in Stata 16.0 (Stata Corporation).
Double data checking
For more assurance on the quality of results, the PI of the study rechecked the full-text data and the analysed data.
Results
Study characteristics
A total of 1461 articles were recruited through the searches, the titles and abstracts of which were assessed by two independent reviewers. Finally, four articles were remained for analysis (Fig. 1).

Fig. 1. Flow diagram of excluded and included studies in this meta-analysis.
These RCT were published between 2005 and 2020. Four studies were performed on both sexes. The sample size of included RCT varied from 134 to 889 participants. A total sample size was 1935 individuals. Studies were conducted in China(Reference Li, Ma and Guo21,Reference Shmuely, Yahav and Samra22) , Iran(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1) and Israel(Reference Zhang, Ma and Pan26). The mean age of participants was between 11 and 50 years. The dosage of cranberry varied from 200 to 560 ml/d, and the duration of intervention ranged from 14 to 90 d across selected RCT.
All studies applied a parallel design, one of them was a multicentre study(Reference Shmuely, Yahav and Samra22) and one trial was a dose–response study(Reference Li, Ma and Guo21). The type of cranberry intake in three trials was the juice form(Reference Li, Ma and Guo21,Reference Shmuely, Yahav and Samra22,Reference Zhang, Ma and Pan26) , two trials administered the capsulated form(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1) and one trial performed the intervention with both(Reference Li, Ma and Guo21). RCT were performed on healthy individuals(Reference Li, Ma and Guo21,Reference Shmuely, Yahav and Samra22,Reference Zhang, Ma and Pan26) and patients with the peptic ulcer disease(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1). The characteristics of four RCT included in the current systematic review and meta-analysis are shown in Table 1.
Table 1. Characteristics of included studies

Assessing for risk of bias
Except for the research of Li et al. (Reference Li, Ma and Guo21), none of the included studies reported adequate information for the methods used for performing random sequence generation.
Only Shmuely et al. (Reference Shmuely, Yahav and Samra22) reported adequate information for the methods used for performing allocation concealment. Also, the study of Seyyedmajidi et al. (Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1) and included RCT had double-blind designs.
The reasons for dropouts were addressed by all studies(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1,Reference Li, Ma and Guo21,Reference Shmuely, Yahav and Samra22,Reference Zhang, Ma and Pan26) .
The studies of Li et al. (Reference Li, Ma and Guo21) and Zhang et al. (Reference Zhang, Ma and Pan26) reported adequate information for the selective reporting(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1,Reference Shmuely, Yahav and Samra22) . Details on the judged risk of each item of bias among the included trials are presented in Figs. 2, 3 and Table 2.
Table 2. The summary of review authors’ judgements on each risk of bias item for included studies based on the Cochrane risk of bias checklist (95 % confidence intervals)

Findings from the systematic review
Among four studies assessing the H. pylori eradication, two studies overall revealed a significant effect of cranberry on H. pylori eradication rate(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1,Reference Zhang, Ma and Pan26) , whereas one study reported a significant effect of cranberry on H. pylori eradication rate in the female but did not reach statistical significance in the men(Reference Shmuely, Yahav and Samra22). Also, one trial revealed a significant effect in the intervention group consumed cranberry juice but those which administrated encapsulated cranberry powder doses did not show any significant effect. In total, four RCT with a total sample size of 1934 subjects were included in the analysis(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1,Reference Li, Ma and Guo21,Reference Shmuely, Yahav and Samra22,Reference Zhang, Ma and Pan26) .
Findings from the meta-analysis
The study results are shown in a forest plot in Fig. 4. The results indicated that the pooled OR was (OR: 1·27; 95 % CI 0·63, 2·58; I 2 = 63·40 %; P = 0·03). Although cranberry supplementation intake had a risk effect on H. pylori eradication, however, this effect was not statistically significant (Fig. 4). One study was discarded for the following reason: children were participated in the trial unlike other studies. But five effect sizes were obtained from the included studies. The results also indicated the moderate between-study heterogeneity (I 2 = 63·40 %; P = 0·03) of the studies.
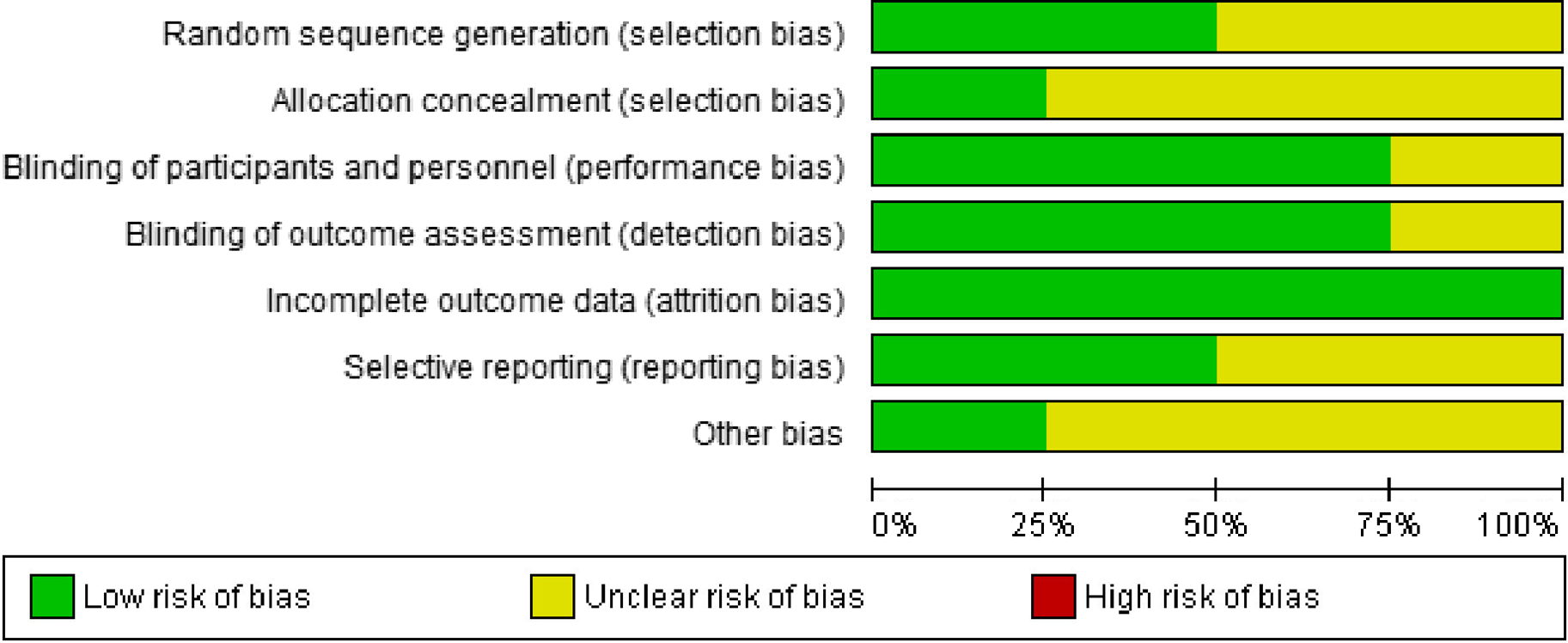
Fig. 2. Risk of bias summary.
Subgroup analysis
In order to detect potential sources of heterogeneity in the meta-analysis, subgroup analyses were performed. Table 3 shows subgroup analysis based on the duration of treatment, the duration of follow-up and the Jadad score by cranberry supplementation intake and H. pylori eradication.
Table 3. Subgroup analysis based on the duration of treatment, the duration of follow-up and the Jadad score
(Odds ratios and 95 % confidence intervals)

The OR for the duration of treatment < 50 and ≥ 50 d were (OR: 1·71; 95 % CI 0·62, 4·70; I 2 = 69·56 %; P = 0·07) (Fig. 5) and (OR: 0·99; 95 % CI 0·34, 2·91; I 2 = 65·30 %; P = 0·05), (Fig. 6) respectively (Table 3). Also, the OR for the duration of follow-up < 100 and ≥ 100 d were (OR: 1·71; 95 % CI 0·62, 4·70; I 2 = 69·56 %; P = 0·07) and (OR: 0·99; 95 % CI 0·34, 2·91; I 2 = 65·30 %; P = 0·05), respectively (Table 3). However, the OR for the Jadad score (3) and the Jadad score (7) were (OR: 1·02; 95 % CI 0·13, 8·13; I 2 = 90·02 %; P = 0·00) and (OR: 1·72; 95 % CI 0·78, 3·78; I 2 = 0·00 %; P = 0·8), respectively (Fig. 7) (Table 3). Due to the low number of studies which reported the Jadad score(Reference Malfertheiner, Megraud and O’Morain5), subgroup analysis was performed but not applicable. Also due to the low number of studies, subgroup analysis was not applicable to other variables.

Fig. 3. Risk of bias summary.

Fig. 4. The meta-analysis results of the effect of cranberry supplementation on Helicobacter pylori.

Fig. 5. The meta-analysis results of the effect of cranberry supplementation on duration of treatment (<50 days and >50 days).

Fig. 6. The meta-analysis results of the effect of cranberry supplementation on duration of follow up (<100 days and >100 days).

Fig. 7. The meta-analysis results of the effect of cranberry supplementation on Jadad score.
Meta-regression
The meta-regression analysis was conducted to evaluate whether the changes in outcomes in response to cranberry supplementation could be associated with the duration of follow-up. The results indicated that H. pylori eradication was not significantly associated with the duration of treatment by cranberry intervention (coefficient = 0·009; P-value = 0·8; se = 0·053; 95 % CI −0·09, 0·11).
Assessment of publication bias
To evaluate publication bias related to the meta-analysis, we can use the metabias function from the Stata and a funnel plot. The results of publication bias are shown in Fig. 8. We saw that the number of studies at the left and the right sides of the funnel plot was almost the same, indicating no detected publication bias. Also, when we did the Egger’s regression tests (P = 0·57; se = 4·94; B = –2·8), no significant publication bias was seen. Using the ‘trim and fill’ method, any significant result was not observed (0·24, 95 % CI −0·466, 0·947) for this meta-analysis.

Fig. 8. Funnel plot of association between cranberry and Helicobacter pylori eradiction.
Sensitivity analysis
Sensitivity analysis showed that the overall effect size regarding the effects of cranberry supplementation on H. pylori eradication did not depend on a single study 1·27 (CI range: 0·63, 2·58). All studies have included this amount. However, in some studies, such as the second, third and fourth studies, the maximum CI has been greater than the overall CI. In the other word, elimination of each RCT at one time or simultaneous elimination of fair and low quality RCT (studies with high risks of bias) did not significantly change the overall estimates of cranberry supplementation on H. pylori eradication (Fig. 9).

Fig. 9. The sensitivity analysis plot for detecting the influence of per included studies on the pooled estimate.
Figure 10 shows the L’Abbé plot for the eradication rate of H. pylori in both the treatment and the control groups. Each circle represents an individual trial and the larger circles represent trials with more participants. The solid diagonal line indicates that the H. pylori eradication rate is equal in the two arms within trials. The dotted line may be called ‘the overall OR line’, as it represents a rate ratio estimated by pooling the results of all studies. The graph shows that the effect size (the OR) of the majority of studies included in the present meta-analysis (four studies) is above 1 and shows the significant effect of supplementation for the eradication of H. pylori.

Fig. 10. L’Abbe plot for Helicobacter pylori eradiction rate in both the treatment and the control groups.
Discussion
Our systematic review and meta-analysis indicated that although cranberry supplementation intake had a risk effect on H. pylori eradication, however, this effect was not statistically significant.
There is a controversy on the cranberry effect on the outcomes of H. pylori eradication. Despite the present and another study(Reference Shmuely, Yahav and Samra22) did not confirm a significant effect of cranberry on H. pylori eradication, some studies have suggested that people who regularly consumed cranberry had significantly H. pylori suppression than others(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1,Reference Howell27) . For example, a study conducted on the effect of cranberry on H. pylori eradication with a standard therapy including lansoprazole, clarithromycin and amoxicillin in patients with the peptic ulcer disease revealed that the addition of cranberry to lansoprazole, clarithromycin and amoxicillin triple therapy for H. pylori has a higher rate of eradication than the standard regimen alone(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1).
Some findings demonstrated the effect of cranberry consumption on H. pylori eradication in demographic subgroups(Reference Gotteland, Andrews and Toledo28) or a specific form of cranberry. So that, animal(Reference Matsushima, Suzuki and Masui29,Reference Nantz, Rowe and Muller30,Reference Haley and Gaddy34) and human studies have shown that the consumption of cranberry juice can be effective in eradicating H. pylori (Reference Zhang, Ma and Pan26,Reference Gotteland, Andrews and Toledo28) . On the other hand, some studies suggested that cranberry had anti-H. pylori properties in in vitro, animal and clinical models which indicated that cranberry consumption alone or administered with antibiotics could reduce H. pylori colonisation but might not fully eradicate it(Reference Moher, Jadad and Nichol25). This properties of cranberry could be due to a high molecular mass constituent derived from cranberry juice inhibited the sialic acid-specific adhesion of H. pylori to human gastric mucus and to human erythrocytes. Also, present proanthocyanidin(Reference Howell20), vitamin C and bioflavonoids with antioxidant properties and a high molecular weight constituent of cranberry juice which may also contribute to the bacteriostatic effect of its juice. The recent study revealed that cranberry juice could inhibit H. pylori adhesion to the human gastric mucosa in vitro. These results were not obtained in powder form(Reference Moher, Jadad and Nichol25,Reference Gotteland, Andrews and Toledo28) .
We found that inconsistent in the results of studies may be due to differences in study designs, study populations or sample sizes, intervention durations, follow-up durations and/or different doses of cranberry. In most of the studies, juice and dried fruit of cranberry were used and maybe the capsule forms are not as effective as the other forms(Reference Seyyedmajidi, Ahmadi and Hajiebrahimi1).
Strengths and limitations
To the best of the authors’ knowledge, the present systematic review and meta-analysis is the first study that evaluates the evidence of cranberry effectiveness on H. pylori. However, the current meta-analysis has some limitations which should be considered. The number of included RCT was low, especially in subgroup analysis.
First, potential publication bias may exist in the observed results since only some established electronic literature databases were searched. Second, due to the lack of detailed information, the quality assessment of the eligible studies may have been influenced by personal judgements.
Third, the heterogeneity of the overall results was moderate in terms of the duration of intervention and the Jadad score, which may have not significantly affected the efficacy of the results. Finally, the results of subgroup analysis in the present meta-analysis based on the duration of treatment, the duration of follow-up and the Jadad score showed that the amount of heterogeneity did not significantly decrease compared with those of the overall analysis. Therefore, these factors (the duration of treatment, the duration of follow-up and the Jadad score) cannot play a significant role as factors in creating heterogeneity between studies. It is possible that other factors, such as how the outcome is measured or important factors not mentioned in the selected initial studies, may play a major role in heterogeneity. It should not be overlooked that the number of studies included in the meta-analysis was small, which can also have a significant impact on not finding sources of heterogeneity.
Conclusion
In conclusion, the results of this systematic review and meta-analysis showed that although there was a positive effect of cranberry supplementation on H. pylori eradication, and it increased the chance of H. pylori eradication by 1·27 times, this relationship was not statistically significant. However, based on the moderate heterogeneity among the included studies, further investigation is needed to evaluate the best dosage, form, duration of follow-up and the optimum effect of cranberry on H. pylori eradication. In the future, more RCT with more subjects and higher quality are still needed.
Acknowledgements
No financial support was provided.
The contributions of the authors were as follows: R. N., Y. M. and M. A. carried out the concept and design and drafting of this study. R. N. and Z. R. searched databases and screened articles. R. N. extracted data. Y. M. and R. N. contributed in the statistical analyses and data interpretations. R. N. carried out writing the report. Y. M., F. S. and M. A. critically revised the manuscript. All authors approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.

















