Obesity is a heterogeneous condition, and individual responses to standardised weight loss protocols are extremely variable and many factors affect the success of a dietary intervention in obesity(Reference Teixeira, Silva and Coutinho1). Different features of meal pattern have been considered in previous studies, including daily meal frequency, circadian distribution of intake(Reference Bellisle2), irregular meal frequency(Reference Farshchi, Taylor and Macdonald3–Reference Farshchi, Taylor and Macdonald5) and omitting breakfast(Reference Farshchi, Taylor and Macdonald6) on weight control, carbohydrate and lipid metabolism.
Current evidence indicates that meal timing may have important effects on body weight, appetite and glucose and lipid metabolism(Reference McHill, Phillips and Czeisler7–Reference Morgan, Shi and Hampton11). It has been indicated that consuming more energy intake in the evening is associated with the risk of obesity(Reference Wang, Patterson and Ang12). A further clinical trial also indicated that a high energy intake at breakfast, with decreased intake at dinner, is beneficial for weight loss and carbohydrate metabolism(Reference Jakubowicz, Barnea and Wainstein13). In our recent intervention study(Reference Madjd, Taylor and Delavari14), we showed that the consumption of a higher energy intake at lunch compared with at dinner may result in greater weight loss and improvement in insulin sensitivity, in women with overweight or obesity after a 12-week weight loss programme.
Considering the timing of specific meals, a recent study showed that late lunch eaters had smaller weight loss than early lunch eaters during a weight loss intervention(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar15). Later lunch is also associated with reductions in resting energy expenditure, fasting carbohydrate oxidation and glucose tolerance(Reference Bandin, Martinez-Nicolas and Ordovas16).
It would thus seem that future weight loss plans should include not only recommendations about energy intake but also the timing of food intake. However, the timing of energy and nutrient intake has changed over time, with a greater proportion of intake later in the day(Reference Almoosawi, Winter and Prynne17). It seems that a busy lifestyle and doing 2–3 shift jobs per day may make it difficult for people to have an early evening meal (EEM) due to long working hours.
The effect of meal timing, specially related to the evening meal, deserves additional study. Epidemiological results indicate a potential negative effect of late evening meals (LEM) on cardio-metabolic health, but clinical intervention studies, which would address this, have been inadequate in range and too varied to draw definitive conclusions and make recommendations(Reference St-Onge, Ard and Baskin18). Also, to our knowledge, no study has examined the effect of a late meal in the evening, compared with an earlier evening meal, on weight loss in women who are overweight or obese during a hypo-energetic diet. Therefore, the primary purpose of this study was to evaluate whether eating a later evening meal, compared to an earlier evening meal, could affect the amount of weight loss in women who are overweight or obese during a weight loss programme.
Materials and methods
Participants
Healthy women were selected between June 2017 and August 2017 from participants who were attending the NovinDiet Clinic, Tehran, Iran, in order to lose weight. Inclusion criteria were female, 18–45 years of age, BMI = 27–35 kg/m2, who were all habitual late evening meal consumers (self-reported usually eating their evening meal at 22.30 hours or later) and were keen to introduce a dietary change to lose weight. All participants were required to be non-smokers, non-shiftworkers, free of history of CVD, stroke, diabetes, liver diseases, kidney diseases, depression, cancer or autoimmune disease. Participants included those who were able to demonstrate that they were able to keep an adequate 4-d food record and reported readiness to safely participate in daily physical activity according to the Physical Activity Readiness Questionnaire(Reference Ferguson19).
Exclusion criteria were pregnancy or lactation during the past 6 months or planned pregnancy in the next 3 months, weight loss ≥10 % of body weight within the 6 months before enrollment in the study, having had bariatric surgery, participating in a research project involving weight loss or physical activity in the previous 6 months, taking medication to lower lipids/cholesterol or that could affect metabolism or change body weight.
The study was approved by the Ethical Committee of The Digestive Research Institute, Tehran University of Medical Science. All participants provided their signed consent prior to study enrollment. This trial was registered at http://www.clinicaltrials.gov/ as NCT03129841.
Study design and interventions
The study was a two-arm, single-blind randomised clinical trial. Included participants were randomly assigned in a 1:1 ratio, after baseline measures, by using a computer-generated random numbers method by the project coordinator. The allocation was obscured from the participants and dietitians until randomisation was disclosed to the study participants at the first intervention clinic appointment. The study groups were the EEM group in which participants ate their evening meal between 19.00 and 19.30 hours and the LEM group in which participants continued to eat their evening meal between 22.30 and 23.00 hours. To control the effects of menstrual cycle on measurements, participants started the study at the same phase of their menstrual cycle. Both groups started a hypo-energetic diet according to the NovinDiet Protocol, which included advice to gradually increase physical activity levels to achieve 60 min of moderate activity on 5 d each week. They were asked to keep a written record of their evening meal time in their log book. Compliance assessment was based on subject adherence to dietary instruction as indicated by the assigned evening meal time. Non-compliance was defined as a deviation of more than 10 % occasions from the recommended evening meal time. The dietitian checked self-reported participant’s time of evening meal in fortnightly visits to the clinic which showed that the participants achieved their time instructed for their evening meal. In addition, participants were asked to record their dietary intake only at week 0, 6 and 12. For these weeks, participants were provided a pedometer and instructed to wear the pedometer for the whole day except when bathing/showering or going to bed and to write their daily step counts and time of the their structured physical activity in their log book. Bi-weekly visits to the dietitian were required in order to measure their weight and promote adherence to the hypo-energetic diet and meal pattern. In addition, a registered dietitian had a telephone conversation with each participant every week during the study to check the adherence to meal pattern, diet and physical activity during the 12-week intervention.
Dietary intervention programme
NovinDiet Clinic is a private weight loss clinic which uses an integrated approach (dietary, behavioural, exercise and medical treatments). Participants in this study did not pay the clinic fees that would otherwise have been required. The programme was designed to enable weight loss of 7–10 % of starting body weight, at a rate of 0·5–1 kg/week over 12 weeks. The individual diet programmes were based on the participants’ food diary records and their food preferences with gradual modification. Participants were assigned to a hypo-energetic diet with a mainly high-carbohydrate, low-saturated-fat dietary pattern (17 % of energy from protein, 23 % from fat (<10 % from saturated fat) and 60 % from carbohydrate – with at least 400 g/d fruits and vegetables to achieve a fibre intake recommendation of 25 g/d(Reference Pem and Jeewon20)). All participants were new to the programme and were not previously under any of NovinDiet Clinic programme.
The diet programme was planned to introduce a 2092−2184 kJ (500−1000 kcal) energy deficit based on estimated energy requirements at the start of the study. Participants consumed 15 % of their energy intake at breakfast and 15% with their snacks, 50 % of daily energy intake at lunch and 20 % at dinner (in both EEM and LEM groups), according to their diet, but there was no particular nutrient composition recommendation for the evening meal.
Besides, participants were encouraged to eat mainly foods with low energy density to achieve satiety, some low-fat dairy products, fibre-rich foods and controlled amounts of high energy dense foods. They were given a plan based on the prescribed macronutrient intake and informed by their food diary record. Plans include common Persian food items. They were also given recipes for the foods within their diets.
The dietary instruction given to the participants was designed to achieve the same energy deficit in the two groups. However, the participants were free living and self-selecting with respect to the time of their dinner meal.
Predominant behaviour change strategies applied included assessing stages of change, goal setting, self-monitoring with food diaries, waist measurements and physical activity(21).
Measurements
Anthropometric measurements were taken at the baseline and after 12 weeks (except weight which was measured at bi-weekly clinic visit and height which was taken only at the screening visit), by the dietitian.
Energy and macronutrient intake at baseline, week 6 and the last week of the intervention (week 12) was analysed using Nutritionist IV software (version 4.1; Hearst). Blood samples of all participants were taken between 07.00 and 09.00 hours, after an overnight fast (8–10 h), at baseline and at 12 weeks for biochemical, cellular and hormonal measurements. Fasting blood samples were collected by venipuncture according to a standard protocol.
Anthropometric measurements
Body weight was taken to the nearest 0·1 kg using a digital calibrated scale (Omron Health Care), with participants wearing light clothing and no shoes. Body height was measured to the nearest 0·1 cm by using a wall mounted stadiometer (SECA) with participants barefoot and in a free-standing position. Waist circumference (WC) was measured with a flexible, non-stretching measuring tape and recorded to the nearest 0·5 cm. WC was measured at the smallest horizontal circumference between the ribs and iliac crest (the natural waist), or, in case of an indeterminable waist narrowing, halfway between the lower rib and the iliac crest(Reference Mason and Katzmarzyk22). BMI was calculated from measured weight in kg divided by the square of height in m.
Blood sample measurements
Blood samples from an antecubital vein via a venipuncture were taken while the participants were in a sitting position, according to the standard protocol(23), and were centrifuged at 2000 g at room temperature within 30–45 min. Blood samples for 2-h postprandial glucose were taken 2 h after ingesting 75 g glucose according to the standard method, and the American Diabetes Association’s criteria were used for excluding diabetes(24). Fasting plasma glucose and 2-h postprandial glucose concentrations were measured with the use of the enzymatic colorimetric method. Insulin was measured by using a radioimmunoassay with 125I-labelled human insulin and a human insulin antiserum in an immunoradiometric assay (Biosource) with a γ-counter system (Gamma I; Genesys). Insulin resistance was evaluated by homoeostasis model assessment of insulin resistance (HOMA-IR), which was calculated by using the following formula(Reference Matthews, Hosker and Rudenski25):
where FPG is fasting plasma glucose. Glycated Hb (HbA1c) was measured by a colorimetric method after an initial separation by ion exchange chromatography (Biosystem). Biochemical analysis of serum total cholesterol (TC), TAG and HDL-cholesterol was carried out on a Selectra E auto analyzer (Vita Laboratory) while following standard procedures for the Pars Azmoon diagnostic kits. LDL-cholesterol was calculated with the use of the Friedewald formula(Reference Friedewald, Levy and Fredrickson26):
Statistical analyses
Baseline values of cardiovascular risk factors (including weight, waist circumference, LDL-cholesterol, HDL-cholesterol, total cholesterol, fasting plasma glucose, TAG, fasting insulin, HOMA-IR, HbA1c and 2-h postprandial glucose) were compared between the EEM and LEM groups using unpaired t tests.
At baseline, distribution was normal for all variables using Kolmogorov–Smirnov test. All participants who were randomly assigned and completed an initial assessment were included in the final results by using an intention-to-treat analysis. Multiple imputations with the use of linear regression were used to impute values that were missing at 12 weeks and were based on the assumption that data were missing at random. To assess the sensitivity of the primary endpoint results (i.e. weight loss) to assumptions about patient dropouts, an additional analysis was considered as adherence throughout the study period (per-protocol analysis). We used ANCOVA to compare the outcomes between the two groups with the baseline values as the covariate. In addition, an ANOVA with repeated measures was used for within-group comparisons.
The primary outcome addressed in this study was the difference in body weight loss after the 12-week weight loss programme. The power calculation was based on the results described by Jakubowicz et al.(Reference Jakubowicz, Barnea and Wainstein13) (α = 0·05, power = 0·9), which were performed based upon an expected difference in weight loss between the diet groups of 5 kg, with a sd of 10 kg, to determine the targeted final sample size (n 43). Anticipating a dropout rate of 40 %, the sample size required was 74. So, eighty subjects were randomly assigned between the two groups of the intervention.
Statistical significance was set at P < 0·05. All data are presented as means and standard deviations unless otherwise stated. All statistical analyses were performed using SPSS 22.0 for Windows (IBM SPSS Statistics for Windows: IBM Corp.).
Results
Baseline characteristics
From 102 people who were initially identified as suitable for the study, twenty participants were excluded because of having exclusion criteria (Fig. 1). The remaining eighty-two participants gave written consent, and then forty were randomly allocated to the EEM group and forty-two to the LEM group. Seventy-five participants completed the 12-week intervention (91 % of the randomly assigned population, Fig. 1). After starting the intervention, a total of seven participants dropped out because they did not wish to continue or due to unexpected changes in their situation. At week 12, the retention rates were 93 % for LEM group and 90 % for EEM group.

Fig. 1. Screening, enrolment, randomisation and follow-up of study participants. FD, food diary; FPG, fasting plasma glucose.
At baseline, there were no statistically significant differences in physical characteristics or biochemical measurements between the intervention groups or between those who completed or did not complete the study once recruited (Table 1).
Table 1. Subject characteristics before the intervention*
(Mean values and standard deviations; percentages)
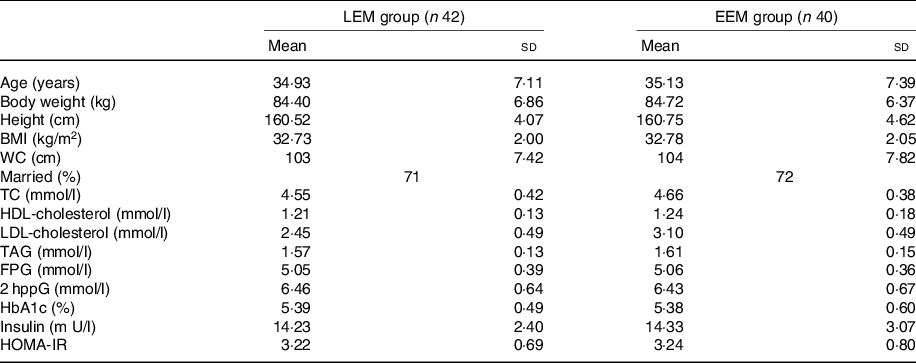
EEM, early evening meal; LEM, late evening meal; WC, waist circumference; TC, total cholesterol; FPG, fasting plasma glucose; 2 hppG, 2-h post prandial glucose; HbA1C, glycated Hb; HOMA-IR, homoeostasis model assessment of insulin resistance.
* Group difference, P > 0·05. There were no significant differences between groups at baseline.
Body weight, BMI and waist circumference
As shown in Table 2, there was a significant weight reduction in each group after 12 weeks (P < 0·001). Also, the primary analysis (intention-to-treat) showed a significant difference in weight reduction between the two groups after 12 weeks (P < 0·001, Table 2, Fig. 2(a)). The per-protocol analysis furthermore indicated a significant greater weight loss of −6·8 (sd 2·01) kg in the EEM group compared with −4·85 (sd 2·3) kg in the LEM group after 12 weeks (P < 0·001, Fig. 2(b)).
Table 2. Anthropometric and blood measurement characteristics in early evening meal (EEM) and late evening meal (LEM) groups before and after the 12-week interventions (n 82)*
(Mean values and standard deviations)

WC, waist circumference; TC, total cholesterol; FPG, fasting plasma glucose; 2 hppG, 2-h post prandial glucose; HbA1c, glycated Hb; HOMA-IR, homoeostasis model assessment of insulin resistance.
* An ANCOVA was used to compare intervention groups (EEM, LEM). Analyses were adjusted for the baseline values. An ANOVA with repeated measures was used for within group comparisons.
† All values are mean differences.
‡ P values represent between-group differences from baseline to 12 weeks after adjustment for the baseline value.
§ Measured by Cohen’s d which is the difference between the two mean changes divided by the pooled standard deviation.
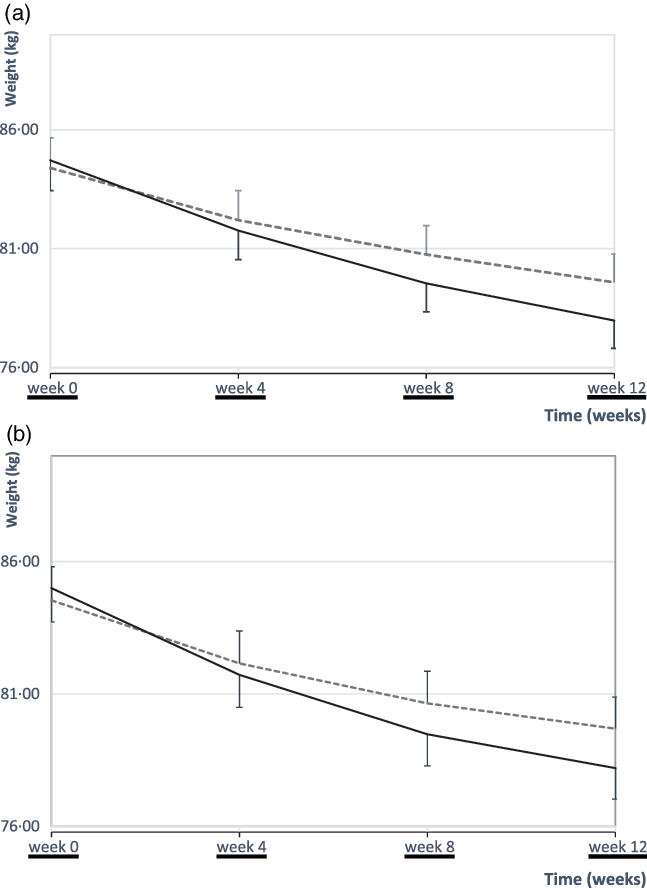
Fig. 2. Mean values with their standard errors body weight over the 12-week intervention. At baseline, there were no differences in body weight between the early evening meal (EEM) (n 40) and late evening meal (LEM) (n 42) groups. A significant weight reduction in each group during the 12-week intervention (time effect, P < 0·001). There was also a significant difference in weight reduction between the two groups after 12 weeks (P < 0·001, ANCOVA). (a) Body weights of all patients who were randomly assigned (EEM group: n 40; LEM group: n 42) (intention-to-treat analysis). (b) Body weights of all patients who adhered throughout the study period (EEM group: n 36; LEM group: n 39) (per-protocol analysis). ![]() , LEM;
, LEM; ![]() , EEM.
, EEM.
The change of BMI in each group was in the expected direction with significant effects over 12 weeks (P < 0·001). However, the drop in BMI was greater in the EEM group than the LEM group after the 12 weeks (Table 2), with a significant difference in BMI changes between the two groups (P < 0·001).
Waist circumference, in both groups, had decreased after the intervention (P < 0·001). There was a significant difference in reduction of WC in the EEM group compared with the LEM group after 12 weeks (P = 0·007).
Lipid profiles
Reductions in TC, LDL-cholesterol and TAG concentration and an increase in HDL-cholesterol were detected over the 12 weeks of study in each group (P < 0·001). Furthermore, there were significant differences in TC (P = 0·038) and TAG in the EEM group compare with the LEM group after the 12 weeks (P < 0·001) (Table 2), but there were no significant differences in LDL- and HDL-cholesterol between groups after 12 weeks (Table 2).
Glucose metabolism measurement
Fasting plasma glucose, fasting serum insulin, 2-h postprandial glucose, HbA1c and HOMA-IR all reduced over time in both groups (P < 0·001). However, between group differences were significant for insulin and HOMA-IR after the 12 weeks of the intervention (Table 2).
There was a significant difference in changes in fasting serum insulin level between the two groups after 12 weeks (P < 0·001) and a significant improvement in insulin sensitivity (measured by HOMA) in the EEM group compared with the LEM group after the 12 weeks (P < 0·001) (Table 2).
Food intake measurement
At baseline, there was no significant difference in energy and macronutrient intakes. Estimated energy intake measurements showed a significant reduction over time in both groups (P time effect < 0·001). As shown in Table 3, there were no significant differences between groups for total energy and macronutrient intakes from baseline to 12 weeks.
Table 3. Self-reported dietary intake in early evening meal (EEM) and late evening meal (LEM) groups at week 0, 6 and 12 of interventions (n 82)
(Mean values and standard deviations)
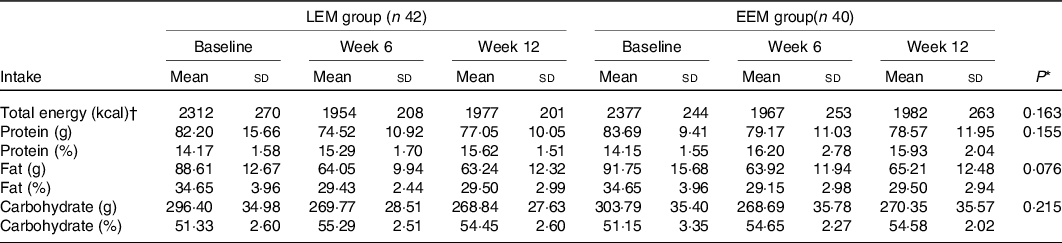
* P values are for EEM relative to LEM group (time × group interaction) by repeated-measures two-way ANOVA.
† To convert kcal to kJ, multiply by 4·184.
Physical activity measurements
As shown in Fig. 3, at baseline both groups had similar mean daily steps of 3990 (sd 651) in the EEM and 3905 (sd 710) in the LEM group. Compared with baseline, both groups had higher mean daily steps over time (P < 0·001 for time effect). However, there were no significant differences between groups for estimated physical activity level during the 12-week intervention (Fig. 3).
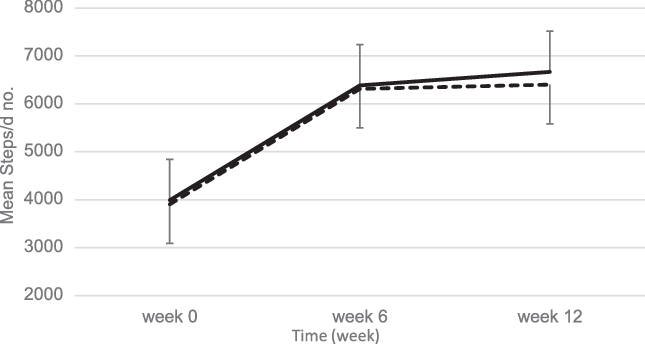
Fig. 3. Mean values with their standard errors step calculations using a pedometer over the 12-week intervention. At baseline, there were no differences in step counts between the early evening meal (EEM) (n 40) and late evening meal (LEM) (n 42) groups. Compared with baseline, both groups had higher mean daily steps over time (P < 0·001 for time effect). There were no significant differences between groups for estimated physical activity level during the 12-week intervention. ![]() , EEM;
, EEM; ![]() , LEM.
, LEM.
Compliance rate of the diet instruction
On average, self-reported compliance (calculated as percentage of days that participants followed dinner time recommendation when filling out the diary) was 93·2 % for the LEM group and 91·8 % for the EEM group, with no significant difference between them.
Discussion
The aim of the current study was to investigate the effect of a different consumption time for the evening meal (EEM v. LEM) on weight loss and carbohydrate and lipid metabolism characteristics in women who are overweight or obese attending a weight loss programme for 12 weeks. We found that eating the evening meal earlier led to more weight loss compared with a LEM during the 12-week weight loss programme. There were also improvements in lipid profiles and insulin sensitivity. To our knowledge, no prior studies have investigated the influence of the timing of consumption of the evening meal on the effectiveness of a weight loss dietary plan.
In the present study, participants in both groups lost weight in a way that was consistent with their meal plans. In intensive clinic-based, behavioural lifestyle modification programmes, 5–10 % weight losses have been observed at 6 months, which would be compatible with the weight losses that we observed during 12 weeks of the current study(Reference Knowler27–29). However, the weight loss was greater in the EEM group than in the LEM group which is in agreement with previous cross-sectional results in which energy intake in the later part of the day, and night-eating syndrome were associated with a higher risk of obesity(Reference Wang, Patterson and Ang12,Reference Colles, Dixon and O’Brien30) . Consistent with the present results, positive effects of early eating during the day on weight loss and anthropometric measures including WC were previously shown(Reference Jakubowicz, Barnea and Wainstein13). However, in that study, a high-energy breakfast was compared with a high-energy dinner in participants with metabolic syndrome and who were overweight or obese. The present study specifically focused on a single meal eaten in the later part of the day compared with an EEM.
Our results are also in agreement with the study which showed that late eaters lost less weight than early eaters(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar15). However, it should be noted that this previous study compared the effects of early v. late lunch eaters not the time of evening meal, which was investigated in the present study.
It can be noted that LEM consumption may cause a greater 24-h energy intake due to no compensation for the energy consumed earlier in the evening. Alternatively, participants in LEM group might eat a bigger evening meal which would have a similar effect if there is no compensation either in intake during the next day or in 24-h energy expenditure. However, the current study failed to find any significant differences in intake of total energy or any of the macronutrients between the late and EEM eaters, despite the significant difference in weight loss. A previous study also showed that late lunch eaters lost less weight and displayed a slower weight loss rate during the 20 weeks of treatment than early eaters, but surprisingly, energy intake was similar between both groups(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar15). In order to establish the underlying mechanisms behind the greater weight loss in the EEM group, future research should assess the potential effects of timing of other meals, physical activity and sleep patterns, psychological aspects and perceived effect on appetite control. Further studies could be also designed to investigate whether there is any threshold time for the evening meal to establish the optimal results for weight management and metabolism.
As a possible explanation for our results, it could be hypothesised that the late eating pattern could influence on circadian genes (SIRT1 and CLOCK loci), which may cause late eaters to be more prone to put on weight and to have less ability to lose it. There is evidence that people carrying minor alleles at both the SIRT1 and CLOCK loci had a significantly weight loss resistance which is associated with late evening preference(Reference Garaulet, Tardido and Lee31). A delayed circadian rhythm in late eaters is also associated with the lower insulin sensitivity(Reference Zhao, Zhang and Zhou32) and metabolic changes(Reference Garaulet and Madrid33) through hormonal changes which leads to be overweight and obesity(Reference Corbalan-Tutau, Madrid and Nicolas34). However, further studies are needed to investigate the actual mechanism behind the results.
In terms of the effects of meal time on glycaemic and lipid profiles, both EEM and LEM groups led to an improvement in cardiometabolic risk factors, which would have been expected given that the participants had lost weight, and showed a reduced WC. However, the EEM group showed a greater reduction in fasting insulin and HOMA-IR in comparison with the LEM group. Our results are consistent with a previous report of relatively impaired insulin responses and lipid tolerance following meals consumed at night(Reference Lund, Arendt and Hampton35), and other studies have shown that insulin sensitivity and glucose tolerance fall gradually during the day with insulin sensitivity reaching the lowest level in the evening(Reference Morgan, Shi and Hampton11,Reference Van Cauter, Shapiro and Tillil36) . Our findings are also in agreement with a previous clinical trial in which, despite similar daily total energy intakes, different energy intakes between breakfast and dinner affected carbohydrate and lipid profiles(Reference Jakubowicz, Froy and Wainstein37).
The findings of the current study may have practical implications, such that consuming an EEM may improve weight loss while attending a weight loss programme. This is in agreement with previous observational evidence that eating the evening meal later is associated with increased risk of obesity(Reference Wang, Patterson and Ang12). A moderate to large effect size(Reference Cohen38) of the measurements indicates that the EEM eaters had a higher weight loss (effect size: 0·87), lower serum TAG (effect size: 0·8) and more improved insulin sensitivity measured by HOMA-IR (effect size: 0·59) relative to the LEM consumers while attending a weight loss plan. However, as this study was done in the specific participants with the instructed meal pattern, further investigations are still needed to offer recommendation for timing of evening meal for general population.
The main strength of the current study is that it was conducted in a free-living population, while participants were on a comprehensive diet and physical activity plan for weight reduction. Participants demonstrated that they were motivated to follow the weight loss plan by achieving the expected level of weight loss. Lastly, providing a free diet plan and weekly telephone call from a dietitian to each participant encouraged them to attend regularly the clinic visits where they were further motivated to adhere to the protocol. However, one limitation is that as a free living study, as opposed to in a closed metabolic unit, full compliance with the dietary protocol could not be guaranteed and 24-h observation was not possible, hence there was a reliance on self-report for key outcome measures, such as food intake(Reference Hill and Davies39). So future studies should involve a design which combines both free-living measures and laboratory assessments of food intake. Moreover, the present study was a short-term intervention, hence does not establish whether the effects noted persist in the longer term, as poor adherence to behaviours recommended in lifestyle interventions is widespread, particularly over the long term(Reference Middleton, Anton and Perri40). Additionally, this study was performed only in premenopausal women with overweight or obesity, and future studies should involve a broader range of participants, for example, men and older women. Furthermore, the weight loss programme involved physical activity recommendations and the time interval between physical activity and the evening meal may affect the response to the timing of the meal. Thus, future studies could also focus on this point. In summary, the results of the current study demonstrate that in the short term earlier eating of the evening meal is more beneficial than later eating for weight loss, insulin sensitivity and lipid profile. Therefore, in people with overweight or obesity, dietary recommendations designed to achieve weight reduction should include advice on time of evening meal intake, in addition to giving recommendations about the overall energy intake. However, the longer-term effects of such changes in the timing of the evening meal need to be evaluated.
Acknowledgements
The authors thank the staff of NovinDiet Clinic, Mansoureh Pahlevani, Leyla Rezaei, Rahil Ahmadi and Nahid Bakhtiari, for their assistance in data collection and Dr Masoud Solaymani and Dr Leila Janani, for their statistical consultation. Thanks also go to Dr Koroush Asadi at the Jaam e Jam Laboratory for the analysis of blood samples.
This study was supported by The School of Life Sciences, The University of Nottingham, UK and The Digestive Disease Research Institute (DDRI), affiliated to Tehran University of Medical Sciences (TUMS).
Experiments in this study were conducted in NovinDiet Clinic, Tehran. A. M.: contributed to the initial study design, study protocol setup, data collection, data analysis and writing of the first draft of the manuscript; M. A. T.: refined the study design and contributed to data interpretation and redrafting of the manuscript, A. M. and M. A. T. contributed to this article as co-first authors. H. R. F.: designed the research, conducted the research, contribution to data interpretation, revision of the manuscript and provided medical supervision; I. A. M.: refined the study design and contributed to data interpretation and redrafting of the manuscript. R. M. and A. D.: provided advice and consultation for the study design, conducted the research. All authors read and approved the final manuscript.
The authors declared no conflicts of interest.











