BMI (kg/m2), as a proxy for body fat percentage, is most commonly used as a device to estimate a healthy body weight for an individual's height. However, this use for individual diagnosis is inappropriate. BMI is more properly a tool for population studies(Reference Keys, Fidanza and Karvonen1, 2).
For the last 30 years, BMI has been used by the WHO as the standard for recording population obesity statistics. Although the subject of debate and modification, for the most part, adult BMI < 18·5 kg/m2 has been considered ‘underweight’, and ≥ 25 kg/m2 but < 30 kg/m2 has been regarded as ‘overweight’, with ≥ 30 kg/m2 being ‘obese’(Reference Inoue and Zimmet3, 4).
Traditionally, the WHO adult BMI categories have been applied uniformly to both the sexes and all ethnicities. This is despite categorisation being based on sedentary Europid individuals of average body composition(Reference Inoue and Zimmet3, 5). Body fat percentage is dependent on such parameters as frame size, muscularity, bone density, physical activity and body proportions, which are related to ethnicity(Reference Rush, Freitas and Plank6). Therefore, in recent years, as evidence has accumulated of population-specific relationships of BMI to body fat percentage and distribution, adoption of different BMI thresholds for different, particularly Asian, populations has been advocated(Reference Inoue and Zimmet3, 4, Reference Rush, Freitas and Plank6, Reference Deurenberg7).
Increasing BMI is associated with increasing adult health risk for ‘lifestyle diseases’ such as diabetes and hypertension(Reference Inoue and Zimmet3). Inherent in the categorisation of BMI is the selection of thresholds at which this risk changes and/or at which intervention is possible or desirable(2). However, this is obscured by the ‘underweight’, ‘normal-weight’, ‘overweight’ and ‘obese’ category nomenclature, and its universal application to disparate populations. Therefore, using diabetes as a marker of morbidity associated with increasing BMI, the present study reports the association of diabetes with BMI in two distinct ethnic groups sharing a common environment, and examines BMI thresholds for the risk of developing diabetes by indigenous Melanesian and Asian Indian populations in the South Pacific island nation of Fiji (837 300 people; 240 700 aged ≥ 40 years, being 50·0 % female, 51·5 % Melanesian Fijian, 42·6 % Indo-Fijian, 5·8 % other ethnicity and 50·6 % rural dwellers).
Methods
The Fiji Eye Health Survey 2009 was a population-based survey of the prevalence, causes and impact of vision impairment and blindness, with particular emphasis on diabetes and diabetic eye disease, for adults aged ≥ 40 years in Fiji.
Sampling plan
The sample frame (188 800 people aged ≥ 40 years; 50·3 % female, 49·4 % Melanesian Fijian, 44·9 % Indo-Fijian, 5·7 % of other ethnicity and 43·2 % rural dwellers) included all eight provinces of Viti Levu, Fiji's main island, where 79·1 % of the population reside. Using an anticipated prevalence of vision impairment of 11 % in the target population, an absolute precision of ± 2·2 % (20 % relative difference), with 95 % confidence, a design effect of 1·4 and a response rate of 80 %, the sample size was determined to be 1354 persons. From the sample frame, thirty-four clusters of forty people were required. Across Viti Levu, the clusters were selected through probability proportionate to size sampling, using national census data.
Pilot
A pilot study was undertaken (forty participants from two clusters, representative of the population to be screened in the main survey) to refine and validate the enquiry, and investigate test–retest reliability. These data were not included in the final survey analysis.
Enumeration
A single survey team visited all clusters during September to November 2009. Using a random process, the team leader identified the first household to be targeted in each cluster. Thereafter, consecutive households were approached, and eligible people enumerated by trained local fieldworkers until the forty participants for that cluster were enrolled. If an eligible person was absent, with no prospect of returning during the team's time in the cluster, the absentee's demographic and socio-economic data were elicited from an available relative in the household or a knowledgeable adult in an adjacent household.
Questionnaire and examination
Enumerated residents amenable to participating attended a central facility, typically a community hall.
A questionnaire, developed in English, translated into Fijian and Hindi, and back-translated to ensure veracity, was used to collect demographic, socio-economic and health data.
Participant barefoot stretch-stature height was measured to the nearest centimetre using a portable stadiometer. Weight, in light tropical clothing and without shoes, was measured to the nearest 0·1 kg using portable scales.
Glycosylated Hb (HbA1c) was determined for each participant using a point-of-care DCA 2000+ analyser (Siemens/Bayer, Munich, Germany).
Study definition
HbA1c ≥ 6·5 % was used to define the presence of diabetes among participants who denied previous diagnosis of the disease by a medical practitioner.
Data analysis
Data were de-identified and entered into a specifically designed database during the survey, with subsequent extensive but random checking for entry integrity. Before analysis, missing and outlier data were checked against the survey forms.
Participants who reported previous personal diagnosis of diabetes were excluded from analyses. Descriptive analysis of the distribution of previously undetected diabetes and of BMI was conducted to investigate mean differences by sex, age group (40–49, 50–59, 60–69 and ≥ 70 years) and ethnicity to inform multivariate analyses. Then, the risk of diabetes was estimated separately for males and females by ethnicity (Melanesian compared with Indian) and WHO BMI categories for adults ( < 18·5, ≥ 18·5 to < 25, ≥ 25 to < 30 and ≥ 30 kg/m2) in a series of logistic regression models to investigate differences, adjusting for age, BMI category and ethnicity. Additional analyses compared empirically derived cut-off points based on the differential distributions of BMI and diabetes for each ethnic group to examine the BMI level for each sex and ethnic group at which there was a significant difference in the risk of diabetes. Descriptive analyses were performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) and OpenEpi 2.3 (www.openepi.com). Logistic regression models were conducted in SAS using PROC GENMOD (SAS Institute Inc., Cary, NC, USA). Statistical significance was accepted at P < 0·05.
Ethical considerations
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving participants were approved by the Fiji National Research Ethics Review Committee (Suva, Fiji), convened by the Fiji Ministry of Health (Suva, Fiji).
Consent was obtained from village chiefs before survey commencement in each cluster. Participants provided written acknowledgement of informed consent before data collection and examinations, including point-of-contact blood analysis. Communications occurred in English, Fijian or Hindi, depending on the participant's preference.
Results
Of the 1892 eligible people enumerated, 1381 participated (73·0 %). However, 27·2 % (139 out of 511) of non-participants were from just five (14·7 %) clusters. Most (63·6 %) non-participants were not at home, with 39·7 % (129 out of 325) of these away for work. Immobility or illness prevented 5·5 % (28 out of 511) attending. Others refused to participate because their eye or vision problem was already being managed (2·3 %) or because there was no perceived problem (1·6 %).
Of the 1381 participants, 222 (16·1 %) claimed a previous personal diagnosis of diabetes had been made by a doctor.
Of the 1159 participants who did not admit having diabetes, 725 were Melanesians, 396 were Indians and thirty-eight were of other ethnicities (excluded from the present analysis). HbA1c was not recorded for twenty-one (2·9 %) and seven (1·8 %) participants of these Melanesians and Indians, respectively: fourteen were because of sporadic omission or analyser error (including sample anaemia), and logistical difficulties at one cluster were responsible for the other fourteen.
Height and/or weight measurements were not recorded for 1·0 % (seven out of 725) of Melanesian and 0·5 % (two out of 396) of Indian participants without self-reported diabetes.
Of the participants for whom HbA1c, height and weight were recorded, and by whom a previous personal diagnosis of diabetes was denied, 697 were Melanesians and 387 were Indians (Table 1). Previously undiagnosed diabetes occurred in 34·0 % (n 369) of these participants. Indians had a significantly higher risk of undetected diabetes than Melanesians in both males (OR 2·99, 95 % CI 1·73, 5·17; P < 0·001) and females (OR 2·26, 95 % CI 1·56, 3·28; P < 0·001), adjusting for BMI and age (Table 2). Participants with a BMI ≥ 25 to < 30 kg/m2 and ≥ 30 kg/m2 also had a significantly higher risk of undetected diabetes compared with those with a BMI ≥ 18·5 to < 25 kg/m2 (Table 2). The risk was substantially higher for males with a BMI ≥ 25 to < 30 kg/m2 (OR 2·35, 95 % CI 1·24, 4·46; P = 0·007) and a BMI ≥ 30 kg/m2 (OR 6·08, 95 % CI 3·06, 12·07; P < 0·001) than for females with the same BMI scores (OR 1·85, 95 % CI 1·11, 3·08; P = 0·027 and OR 2·10, 95 % CI 1·28, 3·44; P = 0·002, respectively).
Table 1 BMI and glycosylated Hb (HbA1c) of 1084 Fiji adults aged ≥40 years who had no previous personal diagnosis of diabetes
(No. of participants, percentages, mean values, standard deviations, and maximum and minimum values)
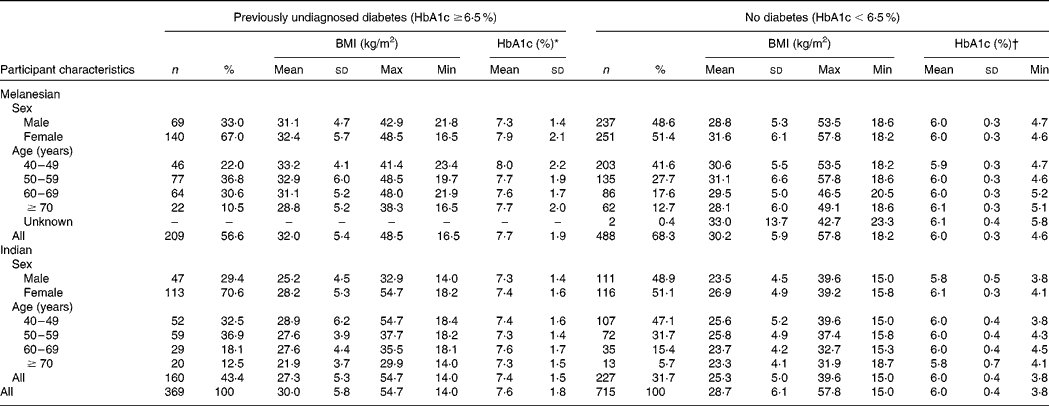
* Minimum by definition of sample was 6·5 %: maximum measurable by the DCA2000+ analyser was 14·0 % (being the case for four (1·9 %) Melanesian and three (1·9 %) Indian Fijians).
† Maximum by definition of sample was 6·4 %.
Table 2 Predictors of diabetes (glycosylated Hb (HbA1c) ≥6·5 %) for 1084 Fiji adults aged ≥40 years for whom this condition was previously undiagnosed
(No. of participants, percentages, odds ratios and 95 % confidence intervals)
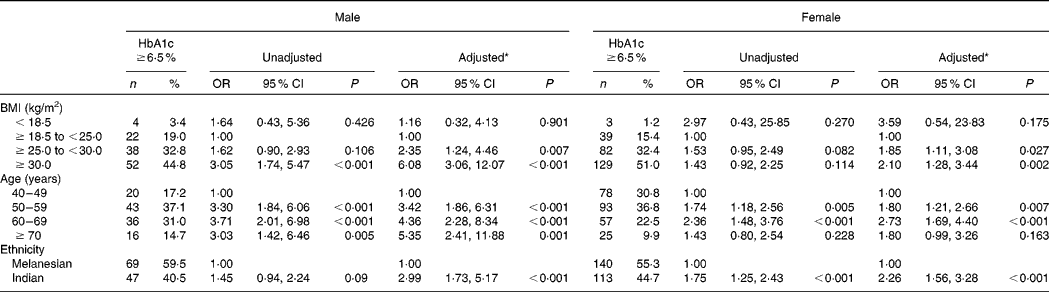
* Adjusted for BMI, age and ethnicity.
Excluding participants with a BMI < 18·5 kg/m2, and adjusting for age, the data suggested that the BMI score discriminated between those at risk of having previously undetected diabetes and those without the disease (Table 3). The BMI threshold above which the risk of having diabetes became statistically significant varied by ethnicity and by sex. For Melanesian Fijians, the thresholds were 25 kg/m2 for males and 32 kg/m2 for females. For Indo-Fijians, these were 24 and 22 kg/m2 for males and females, respectively.
Table 3 Ethnic and sex differences in the risk of Fiji adults aged ≥40 years having diabetes at incremental cut-off points for BMI
(No. of participants, percentages, odds ratios and 95 % confidence intervals)
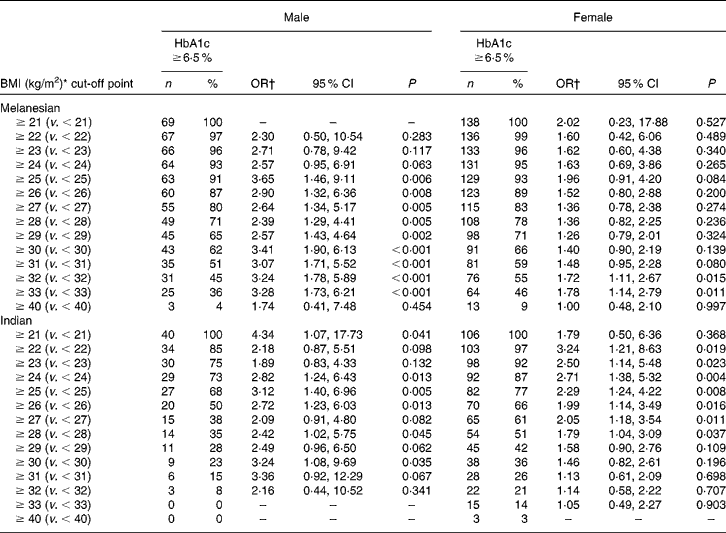
* Participants with a BMI < 18·5 kg/m2 have been excluded.
† OR with 95 % CI adjusted for age.
Discussion
The reason for non-participation in the survey, for the majority, was unlikely to be associated with HbA1c level or BMI. Where participants' HbA1c, height or weight measurements were missing, this was because of sporadic omission or logistics failure. These occurrences were independent of determinants of HbA1c or BMI. Participants who declared a previous personal history of diabetes diagnosis by a doctor were excluded from consideration, because their HbA1c and BMI were likely to have been modified by pharmaceutical treatments and lifestyle management that may have resulted from that diagnosis. Although undiagnosed diabetes may cause weight loss, it was anticipated that the bias so introduced would be minimal, with, as has been accepted in other cross-sectional population surveys exploring the relationship between diabetes and BMI(Reference Craig, Colagiuri and Hussain8), no compromise of the results. Therefore, the sample of 1084 participants used for this investigation was likely to adequately represent HbA1c and BMI for those aged ≥ 40 years among the majority ethnic groups in Fiji.
There is a move towards using presenting HbA1c as a biomarker for the diagnosis of type 2 diabetes mellitus(Reference Gillett9–11). Although not without potential problems(Reference Kilpatrick, Bloomgarden and Zimmet10, Reference Kilpatrick12), as a population screening tool, HbA1c has advantages over the use of plasma glucose concentration, whether fasting or after oral glucose. These include avoiding reliance on self-declared fasting and, when required, waiting for a 2 h value. The efficacy of using HbA1c for population screening has been demonstrated(Reference Rohlfing, Little and Wiedmeyer13, Reference Ginde, Cagliero and Nathan14), as has the use of the point-of-care DCA 2000+ analyser in difficult circumstances(Reference Shemesh, Rowley and Shephard15). Therefore, point-of-contact HbA1c screening with, understanding the inherent limitations(Reference Gillett9, 11, Reference Buchanan, Jha and Matthews16), a threshold of ≥ 6·5 % for the diagnosis of diabetes was chosen for this investigation(11), as it has been for other population studies(Reference Zhang, Saaddine and Chou17).
Increasing BMI is but one risk factor associated with ‘lifestyle’ diseases, including diabetes. Therefore, as anticipated for participants in the present study, increased risk of having diabetes occurred at WHO BMI thresholds of 25 and 30 kg/m2 (Table 2). However, for this disease in this sample, these thresholds did not accurately characterise the distribution of BMI-associated risk, especially when considering Melanesian Fijians and Indo-Fijians separately.
Even a ‘normal’ BMI (WHO: ≥ 18·5 but < 25·0 kg/m2) is associated with some health risk from lifestyle diseases. Therefore, the upper limit of this category is not clinically useful in differentiating those ‘at risk’ compared with those ‘not at risk’. To be useful, the threshold for change from ‘background’ to some level of ‘elevated’ risk (which may be better terminology than ‘normal weight’ and ‘overweight’) needs to accurately represent the risk profile of a particular population for a particular disease or group of diseases. For example, there is growing evidence of increased risk of diseases such as diabetes and hypertension at lower BMI for Asian body types(4, Reference Wen, David Cheng and Tsai18–Reference Odegaard, Koh and Vazquez21), including for Asian Indians(Reference Misra, Chowbey and Makkar22). Certainly, the present investigation suggests that Indo-Fijian females with a BMI of 22–25 kg/m2 (within the ‘normal’ range) were more likely to have diabetes than Melanesian females with a BMI >25 kg/m2 (outside the ‘normal’ range). A reduction of threshold for Indo-Fijians, from the currently accepted BMI of 25 kg/m2, would be consistent with recommendations of the WHO Expert Consultation on BMI in Asian populations(4) and the consensus statement on obesity in Indians issued by physicians in India(Reference Misra, Chowbey and Makkar22). It would also be consistent with recent threshold modifications for Singaporean adults, which, using risk-based taxonomy and category descriptions, acknowledge that a BMI of 18·5–22·9 kg/m2 is associated with a low risk of developing CVD and diabetes, but that at thresholds of 23 and 27·5 kg/m2, this increases to moderate then high risk(23).
There are fewer data available for the island populations of the Pacific. Even so, based on Polynesian data, a BMI threshold of 26 kg/m2 for the transition from normal to overweight has been suggested(Reference Inoue and Zimmet3), and the New Zealand Ministry of Health (Wellington, New Zealand) currently uses thresholds of 26 and 32 kg/m2 for overweight and obesity in Pacific Islander and Maori adults living in New Zealand(Reference Rush, Freitas and Plank6). However, for Fijian Melanesians, sex-specific thresholds may be appropriate. The BMI cut-off point of 25 kg/m2 (Table 3) for Melanesian male risk of diabetes suggested by the present survey was consistent with the WHO categorisation. For females, it was markedly different: no difference in the risk of diabetes was apparent until a BMI of 32 kg/m2. Using thresholds less than this, the data suggest that females with a BMI above and below those cut-off points had no difference in the risk of diabetes. This is despite a BMI of 32 kg/m2 being well beyond the ‘normal’ category.
Although sex threshold disparity was much less pronounced for Indo-Fijians (BMI of 24 kg/m2 for males; BMI of 22 kg/m2 for females; Table 3), it was consistent with observations from India(Reference Misra, Chowbey and Makkar22) and for some other Asian populations. For example, for Hong Kong Chinese(Reference Ko, Chan and Cockram24), different BMI thresholds for the risk of diabetes have been identified: 24·3 kg/m2 for males and 23·2 kg/m2 for females.
The increase in health risk associated with increasing BMI is not uniform for each of the lifestyle diseases. Nor is the risk uniform for all ethnicities, or equal for both the sexes. The present study suggests that, for diabetes at least, consistent with findings from other Asian populations, Indo-Fijians aged ≥ 40 years developed an increase in the risk at BMI lower than the current upper limit of WHO ‘normal’. Living in the same country and overtly same circumstances, the risk profile for Melanesian Fijians was different. This was especially so for Melanesian females, for whom increased risk of diabetes did not occur until BMI was within the WHO ‘obese’ category. Therefore, disaggregating by ethnicity and sex, and applying specific evidence-based thresholds, BMI may become a more discriminating tool for assessing Fijian risk of developing lifestyle diseases such as diabetes, and for prompting action to reduce that risk.
Acknowledgements
The design, implementation and analysis of the Fiji Eye Health Survey 2009 were financially supported by the New Zealand Agency for International Development (Wellington, New Zealand), the Australian Agency for International Development (Canberra, Australia) and The Fred Hollows Foundation New Zealand (Auckland, New Zealand). The authors acknowledge the help of Sanya Baker, Louisa Semmons, Tom Schaefer, Carmel Williams, Losalini Tavaga, Konstanze Fischer-Harder, Biu Sikivou and the Fiji Eye Health Survey 2009 survey team. G. B. and J. R. participated in the survey design and implementation, data preparation and analysis, and manuscript preparation. A. P. contributed to data analysis and manuscript preparation. L. M., J. S. and M. Q. Q. were involved in survey implementation and manuscript preparation. All authors approved the final manuscript. The authors have no conflicts of interest.







