Numerous epidemiological and experimental studies suggest that changes in the composition of the macronutrients of the diet are important environmental determinants in the prevention or improvement of several metabolic disorders included in the ‘so-called’ metabolic syndrome such as type 2 diabetes, insulin resistance, hypertension, obesity and CVD(Reference Cheal, Abbasi, Lamendola, McLaughlin, Reaven and Ford1–Reference Mori and Beilin3). In this regard, a significant body of evidence indicates that the dietary intake of marine PUFA (fish oil rich in 20 : 5n-3 (EPA) and 22 : 6n-3 (DHA)), involved in many biological processes, appears to play an important role against the adverse effects of this syndrome(Reference Clarke2, Reference Connor4–Reference Lombardo and Chicco6). On the other hand, α-linolenic acid (ALA; 18 : 3n-3), the precursor of long-chain n-3 fatty acids which can be converted to EPA and DHA, has received less attention. Therefore, it is possible that dietary ALA could exert similar physiological effects as in the case of EPA and DHA from dietary fish oil(Reference Simopoulos7). Previous results of ALA have not always been consistent depending on the experimental conditions such as the type and amount of oil, or feeding period(Reference Kawashima and Kozuka8–Reference Ihara, Umekawa, Takahashi and Furuichi11). Kim et al. (Reference Kim, Choi and Choi12) and Kim & Choi(Reference Kim and Choi13) reported that in normal rats dietary perilla oil (rich in ALA) was effective both in suppressing fatty acid synthase activity and decreasing hepatic and plasma TAG levels compared with rats fed maize oil rich in linoleic acid (LA; 18 : 2n-6). These effects were associated with the increase of hepatic microsomal EPA and DHA contents. Other studies failed to show significant differences between LA and ALA in the hepatic lipogenesis key enzyme activities and fatty acid synthesis(Reference Javadi, Geelen, Lemmeus, Lankhorst, Schonewille, Terpstra and Beynen14). One of the richest botanical sources of ALA is chia seed (Salvia hispanica L.)(Reference Weber, Gentry, Kohlhepp and McCrohan15, Reference Bushway, Wilson, Houston and Bushway16). Chia, which is also rich in fibre and minerals, was a key component in the diet of many pre-Columbian peoples in America, including the Mayan and the Aztec civilisations. Recently Ayerza & Coates(Reference Ayerza and Coates17) demonstrated that ground chia seed given to normal rats as a source of fat in the diet was able to reduce plasma TAG and total cholesterol levels, and increased HDL-cholesterol.
Several investigations have demonstrated that normal rats fed a high-carbohydrate (particularly fructose or sucrose) diet for 3–5 weeks develop hypertriacylglycerolaemia, increased plasma NEFA levels, hyperinsulinaemia, impaired glucose tolerance and decreased insulin action(Reference Lombardo and Chicco6, Reference Reaven, Shaffrir and Renold18–Reference Bezerra, Ueno, Silva, Tavares, Carvalho and Saad22). However, we have shown that the metabolic and hormonal profile obtained in rats chronically fed a sucrose-rich diet (SRD) depends on both the length of time and the amount of sucrose administered(Reference Gutman, Basílico, Bernal, Chicco and Lombardo23). In this regard, Chicco et al. (Reference Chicco, Basabe, Karabatas, Ferraris, Fortino and Lombardo24, Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25) reported that when the diet was extended up to 15 to 40 weeks, a steady state of hypertriacylglycerolaemia, elevated plasma NEFA levels, moderate hyperglycaemia, insulin resistance and altered patterns of glucose-stimulated insulin secretion from perifused islets could be observed. In addition to dyslipidaemia, moderate overweight, visceral adiposity and a substantial increase of TAG storage in non-adipose tissues (for example, liver, pancreas, skeletal and heart muscles) have been demonstrated(Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25, Reference Pighin, Karabatas, Rossi, Chicco, Basabe and Lombardo26). All these metabolic effects induced by feeding a long-term SRD closely resemble those found in humans with overweight and/or type 2 diabetes. Interestingly, the addition of dietary fish oil to the SRD instead of the usual oil in the rat diet for a short period of time (2–4 weeks) was able to prevent the development of hypertriacylglycerolaemia and the abnormal glucose homeostasis(Reference Lombardo and Chicco6). Moreover, earlier studies(Reference Lombardo and Chicco6, Reference Pighin, Karabatas, Rossi, Chicco, Basabe and Lombardo26) have shown that by shifting the source of fat in the diet (maize oil replaced by fish oil), the dyslipidaemia and insulin resistance induced in rats fed a long-term (15–40 weeks) SRD are reversed. So far, only a few studies in the literature have examined the effectiveness of dietary ALA on the SRD-induced insulin resistance in rats. Recently, Ghafoorunissa et al. (Reference Ghafoorunissa, Ibrahim and Natarajan27) showed that in weanling rats fed a SRD, the substitution of one-third of dietary 18 : 2n-6 with 18 : 3n-3 PUFA during 3 months resulted in lowered blood lipid levels and partially corrected sucrose-induced insulin resistance.
Therefore, the present study investigates the possible benefits of the dietary intake of chia seed (rich in ALA) in the prevention, improvement and/or reversal of the dyslipidaemia and insulin resistance induced in normal rats by feeding them a SRD. To achieve these goals, two kinds of experiments were designed: (i) to investigate the prevention of the development of dyslipidaemia and abnormal glucose homeostasis; (ii) to analyse the effectiveness of chia seed in improving or reversing the metabolic abnormalities described above. In both experimental designs, several aspects of lipid and glucose metabolism were studied, focusing particularly on the relationship between the lipid-lowering actions of chia seed and the concomitant effects on glucose homeostasis and whole-body peripheral insulin sensitivity (euglycaemic–hyperinsulinaemic clamp).
Materials and methods
Animals
Male Wistar rats (initial weight 180–190 g; National Institute of Pharmacology, Buenos Aires, Argentina) were maintained with unrestricted access to water and food, under controlled temperature (22 ± 1°C), humidity and air-flow conditions, with a fixed 12 h light–dark cycle (light 07.00 to 19.00 hours). They were initially fed a standard laboratory chow (Ralston Purina, St Louis, MO, USA) containing by weight (per 100 g): 63 g starches, 3·5 g fat, 6·0 g fibre and 5 g vitamin mixture and salt mixture. After 1 week of acclimatisation, the rats were randomly assigned to one of the two experimental protocols as described below. The experimental protocols were approved by the Human and Animal Research Committee of the School of Biochemistry, University of Litoral, Argentina.
Dietary manipulations
Experimental design 1
Seventy-two rats were randomised into three groups and fed an assigned diet for 3 weeks (Table 1). The first group of rats received a semi-synthetic diet containing maize starch (60 % energy), protein (17 % energy) and maize oil as the source of fat (23 % energy) (control diet). The other two groups received the same semi-synthetic diet with sucrose as the carbohydrate and fat provided by maize oil (SRD) or by chia seed (SRD+chia).
Table 1 Composition of the experimental diets*
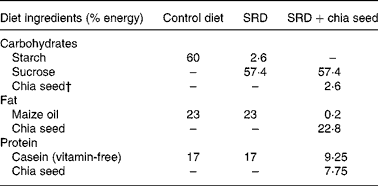
SRD, sucrose-rich diet.
* Diets are based on the AIN-93 diet. All diets contain by weight: salt mix, 3·5 % (AIN-93M-MX(Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25)); vitamin mix, 1 % (AIN-93 VX(Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25)); choline chloride, 0·2 %; methionine, 0·3 %; fibre, 12 %. The SRD+chia was balanced in fibre and salt mix according to the amount of each one in the chia seed provided by the manufacturer.
† Chia seed (Salba; Salvia hispanica L.): 362 g/kg diet. Chia composition (g/100 g chia seed): carbohydrate, 37·45; insoluble fibre, 81 % of total carbohydrate; fat, 30·23; protein, 21·19. Mineral composition (mg/100 g chia seed): Na, 103·15; K, 826·15; Ca, 589·60; Fe, 11·90; Mg, 77·0; P, 604·0; Zn, 5·32; Cu, 1·66; Mn, 1·36.
Experimental design 2
Ninety-six rats were divided into two groups and fed for 3 months with the control diet (n 24) or SRD (n 72) previously described in Experimental design 1. At that time, rats in the SRD groups were randomly subdivided into three subgroups. The first subgroup was immediately killed for each procedure as described below. The rats in the second subgroup continued with the SRD up to 5 months of feeding. The third subgroup received the SRD+chia seed as the source of dietary fat for the next 2 months. The control group was fed with the control diet throughout the experimental period. The fibre, vitamin mix and salt mix contents of each diet were similar. The carbohydrate, protein and fibre content in the feed of the SRD+chia group was balanced taking into account the amount of these nutrients present in the chia seed. The composition of the chia seed was provided by the supplier (Table 2). The preparation and handling of the diets have been reported elsewhere(Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25). The food in the animal cages was shaded from light and changed every day. All diets provided approximately 18·00 kJ/g chow. The weight of each animal and the energy intake were recorded twice per week during the experimental period in all groups and subgroups of rats. On the day of the experiment, food was removed at 07.00 hours (the end of the dark period) unless otherwise indicated, and the experiments were performed between 07.00 and 09.00 hours. At least six rats from the three dietary groups were used in each procedure. They were anaesthetised with intraperitoneal pentobarbital Na (60 mg/kg body weight). Blood samples were obtained from the jugular vein and rapidly centrifuged; plasma was either immediately assayed or stored at − 20°C. The liver, skeletal muscle (gastrocnemius), epididymal and retroperitoneal adipose tissue were totally removed, weighed and immediately frozen and stored at the temperature of liquid N2.
Table 2 Maize oil and chia seed fatty acid composition (g/100 total fatty acids)
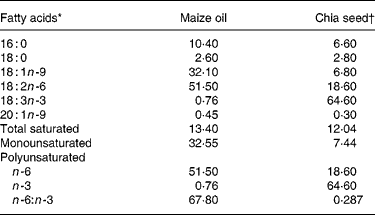
* Minor components made content up to 100 %.
† Salba; Salvia hispanica (Agrisalba SA, Buenos Aires, Argentina).
Analytical methods
Plasma TAG, NEFA, total cholesterol, HDL-cholesterol and glucose levels were determined by spectrophotometric methods as previously described(Reference Lombardo, Chicco, D'Alessandro, Martinelli, Soria and Gutman28). The immunoreactive insulin was measured by the method proposed by Herbert et al. (Reference Herbert, Lau, Gottlieb and Bleicher29). The insulin assays were calibrated against rat insulin standard (Novo Nordisk, Bagsværd, Denmark). The TAG content was determined in homogenates of the frozen liver and gastrocnemius muscle(Reference Lombardo, Chicco, D'Alessandro, Martinelli, Soria and Gutman28).
Total serum lipids were extracted according to the method described by Folch et al. (Reference Folch, Lees and Sloane Stanley30) in the presence of butylated hydroxytoluene as an antioxidant. Serum fatty acids were analysed by capillary GC in a high-resolution column (60 m and 0·25 mm internal diameter) with a KNK 3000, HRGC (Konik Instruments, Miami, FL, USA) after their derivatisation as methylated fatty acids(Reference Lombardo, Chicco, D'Alessandro, Martinelli, Soria and Gutman28). Fatty acids were identified by retention time in comparison with known standards.
TAG secretion rate
The TAG secretion rate (TGSR) was evaluated in fasting rats (16–18 h) by blocking the removal of plasma TAG with Triton WR 1339 (600 mg/kg body weight) dissolved in 0·9 % NaCl. The TGSR was calculated from the linear increase of TAG v. time, following the procedure previously described by Lombardo et al. (Reference Lombardo, Chicco, D'Alessandro, Martinelli, Soria and Gutman28).
Intravenous fat tolerance test
The intravenous fat tolerance test was performed in rats fasted for 16–18 h by injecting intravenous Intralipid® (Intralipid® 10 % at 0·1 ml/100 g body weight), a soyabean oil fat emulsion that has been found to be a useful tracer for the study of the fractional turnover rate of circulating TAG(Reference Rössner31). The disappearance of Intralipid® in plasma was measured by nephelometry. The values were plotted on a semi-logarithmic scale v. time. The first-order rate constant (K2) of elimination of fat emulsion from the bloodstream (fractional removal rate) was calculated by the least-squares method as described elsewhere(Reference Lombardo, Chicco, D'Alessandro, Martinelli, Soria and Gutman28).
Euglycaemic clamp studies
Whole-body peripheral insulin sensitivity was measured using the euglycaemic–hyperinsulinaemic clamp technique as described elsewhere(Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25). Briefly, after 5 h of food deprivation, six rats from each dietary group were anaesthetised, a blood sample was withdrawn and glucose and insulin levels were assessed. Afterwards, an infusion of highly purified porcine neutral insulin (Actrapid; Novo Nordisk, Bagsværd, Denmark) was administered at 0·8 units/(kg × h) for 2 h. Glycaemia was maintained at a euglycaemic level by infusing glucose (200 g/l) at a variable rate. The glucose infusion began 5 min after the insulin infusion started. The blood glucose concentration was measured using a Glucomether Analyser (Boehringer Mannheim, Indianapolis, IN, USA) within 2 min after the samples were obtained. The glucose infusion rate during the second hour of the clamp study was taken as the net steady state of the whole-body glucose. In all studies, blood samples (0·3 ml) for insulin determination(Reference Herbert, Lau, Gottlieb and Bleicher29) were obtained at 60, 90 and 120 min. Details of the methodology have been provided elsewhere(Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25).
Statistical analysis
Sample sizes were calculated on the basis of measurements previously made with rats fed either a control diet or a SRD(Reference Lombardo, Chicco, Mocchiutti, Rodi, Nusimovich and Gutman20, Reference Gutman, Basílico, Bernal, Chicco and Lombardo23, Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25, Reference Pighin, Karabatas, Rossi, Chicco, Basabe and Lombardo26), considering an 80 % power. Results were expressed as mean values with their standard errors. Statistical comparisons were done transversely between different dietary groups. The statistical significance between groups was determined by one-way ANOVA, with one factor (diet) followed by the inspection of all differences between pairs of means by the Newman–Keuls test(Reference Snedecor and Cochran32). Differences having P values lower then 0·05 were considered to be statistically significant. In all cases the interclass correlation coefficients were at least 0·73.
Results
Energy intake and body-weight gain
Energy intake and body weight were carefully monitored in all groups of rats throughout both experimental periods. Fig. 1 (a) depicts a comparable weight gain and energy intake in the rats fed the control diet, the SRD and the SRD + chia seed during the first 3 weeks on the diets (Experimental design 1). As shown in an earlier study(Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25), comparable weight gain and energy intake were also recorded in the SRD and control diet rats during the first 3 months on their respective diets (Experimental design 2). However, a significant increase in weight gain and energy intake was observed in the rats that continued with the SRD up to 5 months. Even though a similar energy intake was recorded in both the SRD and SRD+chia seed groups during the last 2 months of the experimental period (3 to 5 months), the weight gain in the rats fed the SRD+chia seed was slightly lower than in the group of rats fed the SRD (Fig. 1 (b)).

Fig. 1 Body weight and energy intake of rats fed a control diet (□), sucrose-rich diet (SRD; ![]() ) or the SRD+chia seed (Salvia hispanica L.; ■). Chia seed replaced maize oil as the source of dietary fat in the SRD from the beginning of the experiment and during 3 weeks (a). Chia seed replaced maize oil as the source of dietary fat in the SRD from 3 to 5 months of the experiment (b). Values are means for twenty-four animals per group, with standard errors represented by vertical bars. a,b Mean values in a column with unlike superscript letters were significantly different (P < 0·05) when one variable at a time was compared by the Newman–Keuls test. * Mean value was significantly different from those of the SRD and SRD+chia groups (P < 0·05).
) or the SRD+chia seed (Salvia hispanica L.; ■). Chia seed replaced maize oil as the source of dietary fat in the SRD from the beginning of the experiment and during 3 weeks (a). Chia seed replaced maize oil as the source of dietary fat in the SRD from 3 to 5 months of the experiment (b). Values are means for twenty-four animals per group, with standard errors represented by vertical bars. a,b Mean values in a column with unlike superscript letters were significantly different (P < 0·05) when one variable at a time was compared by the Newman–Keuls test. * Mean value was significantly different from those of the SRD and SRD+chia groups (P < 0·05).
Fad pad and liver weights
As shown in Table 3, after 3 weeks on diet (Experimental design 1), epididymal and retroperitoneal fat pad weight, total or relative to 100 g body weight, were comparable in the three dietary groups. In long-term feeding (5 months; Experimental design 2) and in agreement with previous results(Reference Soria, D'Alessandro and Lombardo33), the epididymal and retroperitoneal fat depot of the control diet-fed rats grew accordingly with the increase of body weight. In the SRD-fed group, there was a significant increase in both epididymal and retroperitoneal fat pad weights either expressed as total or relative weight compared with the age-matched controls fed the control diet. When chia seed replaced maize oil as the source of dietary fat in the SRD, only a significant decrease of relative to total weight in both fat pads was observed. In the SRD+chia seed group total weight of epididymal and retroperitoneal tissue showed a slight, albeit not significant, decrease compared with the rats fed the SRD. No differences in liver weights (total or relative to 100 g body weight) were observed in the three dietary groups either in the short- or long-term studies (data not shown).
Table 3 Total and relative white adipose tissue weights of rats fed the control diet, sucrose-rich diet (SRD) or the SRD+chia seed (Salvia hispanica L.)
(Mean values with their standard errors for six animals per experimental group)
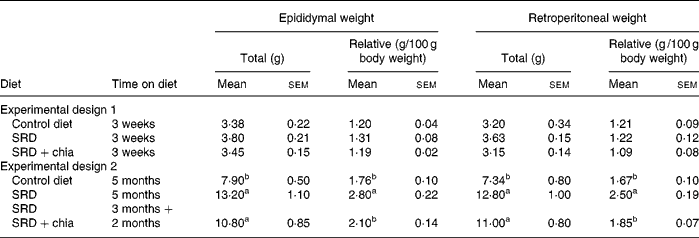
a,b Mean values in a column with unlike superscript letters were significantly different (P < 0·05) when one variable at a time was compared by the Newman–Keuls test in each experimental design.
Plasma metabolite levels
After a 3-week feeding period (experimental design 1), both plasma TAG and NEFA were significantly higher in the SRD-fed rats than in the control diet-fed rats. The presence of chia seed in the SRD group was able to prevent these alterations. On the other hand, while total cholesterol was similar in the SRD- and control diet-fed rats, there was a significant decrease (P < 0·001) in the SRD+chia seed group (Table 4). Moreover, the HDL-cholesterol:total cholesterol ratio was similar in the three dietary groups. Values were as follows for six rats: 61·60 (sem 2·40) % in the control diet group, 59·50 (sem 1·20) % in the SRD group and 66·20 (sem 2·60) % in the SRD+chia seed group.
Table 4 Plasma TAG, NEFA and total cholesterol levels of rats fed the control diet, sucrose-rich diet (SRD) or the SRD+chia seed (Salvia hispanica L.)
(Mean values with their standard errors for six animals per experimental group)
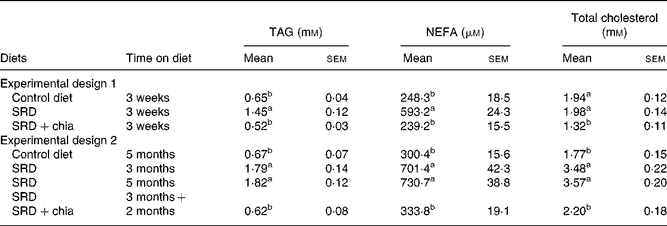
a,b Mean values in a column with unlike superscript letters were significantly different (P < 0·05) when one variable at a time was compared by the Newman–Keuls test in each experimental design.
A different picture emerged after a long-term feeding period (Experimental design 2). Plasma TAG, NEFA and total cholesterol levels were higher either after 3 or 5 months on the SRD than in the control diet-fed rats (P < 0·01) (Table 4). When the source of dietary fat was replaced in the SRD group for the last 2 months on the diet (SRD+chia seed), plasma TAG, NEFA and total cholesterol levels reached values similar to those of the control diet-fed animals. Furthermore, the HDL-cholesterol:total cholesterol ratio that was significantly decreased in the SRD-fed rats was restored to normal values after the SRD+chia seed. Values were as follows for six rats: 57·3 (sem 3·3) % in the control diet group, 32·0 (sem 3·7) and 31·9 (sem 2·9) % in the SRD group at 3 and 5 months, respectively, and 58·1 (sem 3·5) % in the SRD+chia seed group (P < 0·001 in SRD-fed rats at 3 and 5 months v. control diet- and SRD+chia seed-fed rats).
Liver triacylglycerol, triacylglycerol secretion rate and fractional removal rate of fat emulsion
The rats fed the SRD during 3 weeks showed a significant increase of ‘in vivo’ TGSR and a decrease of fractional removal rate (K2; %/min) of the intravenously injected fat emulsion (Intralipid®) compared with the age-matched controls fed the control diet (Figs. 2 (a) and (b)). This was accompanied by a significant increase of liver TAG content (Fig. 2 (c)). A similar picture of the above parameters was observed after 3 or 5 months on the SRD (Figs. 2 (d), (e) and (f)). However, the liver TAG contents in the long-term SRD-fed rats (3 or 5 months) were significantly higher than those recorded after 3 weeks on the SRD (Figs. 2 (c) and (f)).

Fig. 2 TAG secretion rate (TGSR) (a and d), fractional removal rate of fat emulsion (K2) (b and e) and liver TAG (c and f) of rats fed a control diet (□), sucrose-rich diet (SRD) or the SRD+chia seed (Salvia hispanica L.). Chia seed replaced maize oil as the source of dietary fat in the SRD from the beginning of the experiment and during 3 weeks (a, b and c). Chia seed replaced maize oil as the source of dietary fat in the SRD from 3 to 5 months of the experiment (d, e, f). Values are means for six animals per group, with standard errors represented by vertical bars. (![]() ), SRD for 3 weeks; (
), SRD for 3 weeks; (![]() ), SRD+chia for 3 weeks; (
), SRD+chia for 3 weeks; (![]() ), SRD for 3 months; (
), SRD for 3 months; (![]() ), SRD for 5 months; (
), SRD for 5 months; (![]() ), SRD for 3 months then SRD+chia for 2 months. * Mean value was significantly different from those of the control and SRD+chia groups (P < 0·05). † Mean value was significantly different from that of the control group (P < 0·05).
), SRD for 3 months then SRD+chia for 2 months. * Mean value was significantly different from those of the control and SRD+chia groups (P < 0·05). † Mean value was significantly different from that of the control group (P < 0·05).
The substitution of maize oil by chia seed in the SRD (SRD+chia seed) during 3 weeks (Experimental design 1) significantly decreased TGSR (P < 0·05) and increased the removal rate of TAG from the circulation (P < 0·05). Moreover, liver TAG content was completely normalised (Fig. 2 (c)). On the other hand, when chia seed replaced maize oil for the last 2 months in the SRD (Experimental design 2), TGSR and fractional removal rate (K2; %/min) reached values similar to those recorded in the control diet-fed rats (Figs. 2 (d) and (e)). However, although liver TAG content significantly decreased (P < 0·05), values were still above those of the control diet-fed group (Fig. 2 (f)).
Plasma glucose and insulin levels and glucose infusion rate
After a 3-week feeding period (Experimental design 1), plasma glucose levels were similar in the three dietary groups. Moreover, the hyperinsulinaemia induced by the SRD previously reported elsewhere(Reference Lombardo, Chicco, Mocchiutti, Rodi, Nusimovich and Gutman20, Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25) and confirmed in the present findings was partially decreased in animals fed the SRD+chia seed (Table 5). When the SRD diet was continued up to 3 or 5 months, in both periods plasma glucose levels were greater compared with the control diet-fed rats. However, plasma insulin levels were similar to those observed in the control diet group (Table 5). When the source of dietary fat was replaced in the SRD group for the last 2 months on the diet (SRD+chia seed), plasma glucose returned to normal values without any significant changes in plasma insulin levels in all dietary groups.
Table 5 Plasma glucose and insulin levels and glucose infusion rate (GIR) of rats fed the control diet, sucrose-rich diet (SRD) or the SRD+chia seed (Salvia hispanica L.)
(Mean values with their standard errors for six animals per experimental group)
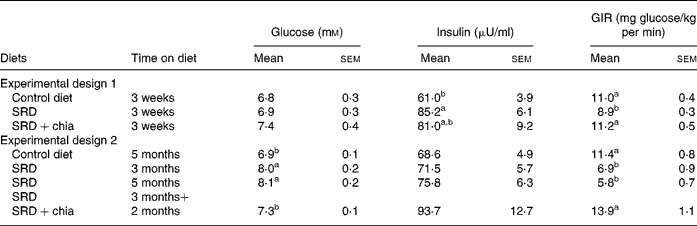
a,b Mean values in a column with unlike superscript letters were significantly different (P < 0·05) when one variable at a time was compared by the Newman–Keuls test in each experimental design.
To assess the effect of chia on whole-body peripheral insulin sensitivity (insulin resistance), a euglycaemic–hyperinsulinaemic clamp study was performed at the end of both experimental designs (1 and 2). Blood glucose was clamped at 5·5–6·0 mm. Postprandial glucose concentration at 5 h before the clamp was as follows for Experimental design 1 (3 weeks of feeding; six rats per group): 5·30 (sem 0·06) mmol/l in the control diet group, 5·50 (sem 0·08) mmol/l in the SRD group and 5·45 (sem 0·05) mmol/l in the SRD+chia seed group. For Experimental design 2 (5 months of feeding; six rats per group) postprandial glucose concentration at 5 h before the clamp was: 5·28 (sem 0·07) mmol/l in the control diet group, 7·75 (sem 0·12) mmol/l in the SRD group and 5·32 (sem 0·08) mmol/l in the SRD+chia seed group. Postprandial glucose levels in the group of rats fed the SRD during 3 months were similar to those recorded at 5 months of feeding. In all dietary groups, plasma insulin levels were similar to those obtained at the end of the dark period in both sets of experiments (data not shown). Table 5 shows a significant decrease of the glucose infusion rate in the SRD-fed groups compared with the control diet-fed group, either after 3 weeks or 3 and 5 months of feeding. However, when chia seed replaced maize oil as the dietary fat in the SRD, the glucose infusion rate returned to values similar to those recorded in the control diet-fed rats in both short- and long-term experimental feeding. There were no changes in packed cell volume from the start to the end of the clamp (data not shown).
Muscle TAG content
No changes in muscle TAG contents were recorded in the three dietary groups after a 3-week feeding period (Experimental design 1) (data not shown). However, after a long-term period (3 or 5 months) on the SRD, the TAG content within the gastrocnemius muscle was significantly increased compared with the control diet-fed group. When chia seed replaced maize oil as the source of dietary fat in the SRD for the last 2 months of the experimental period, the TAG content of gastrocnemius muscle was similar to that observed in the control diet-fed rats. Values were as follows for six rats per group: 3·08 (sem 0·10) μmol/g wet tissue in the control diet group, 5·06 (sem 0·13) μmol/g wet tissue in the SRD-fed group after 3 months, 6·97 (sem 0·10) μmol/g wet tissue in the SRD-fed group after 5 months and 3·10 (sem 0·15) μmol/g wet tissue in the SRD+chia seed group (P < 0·05; SRD after 3 months and SRD after 5 months v. control diet or SRD+chia seed group).
Serum fatty acid analysis
Table 6 shows plasma fatty acid composition, as a percentage of total fatty acid content, in the rats fed the control diet, SRD or SRD+chia seed at the end of 3 weeks of feeding. Both palmitic (16 : 0) and stearic (18 : 0) fatty acids as well as total SFA were similar between the dietary groups. The percentage of polyunsaturated n-6 LA was also similar between the different diets while arachidonic acid (20 : 4), a metabolite of LA, was significantly lower in the SRD+chia seed-fed rats. Total polyunsaturated n-6 fatty acids were significantly lower in the SRD+chia seed-fed group compared with the control diet- and SRD-fed rats. When chia seed replaced maize oil as the source of dietary fat in the rats fed the SRD, the serum levels of the n-3 PUFA, ALA, EPA and DHA, were significantly higher compared with those obtained in the control diet- and SRD-fed rats. Moreover, total n-3 PUFA and the n-3:n-6 ratio were significantly higher in the serum of the rats fed chia seed. The serum fatty acid composition at the end of the long-term feeding period (Experimental design 2) was similar to those described above in the three dietary groups (data not shown).
Table 6 Fatty acid composition of plasma lipids (g/100 g total fatty acids) of rats fed the control diet, sucrose-rich diet (SRD) or the SRD+chia seed (Salvia hispanica L.)
(Mean values with their standard errors for six animals per experimental group)

nd, Not detected.
a,b,c Mean values in a column with unlike superscript letters were significantly different (P < 0·05) when one variable at a time was compared by the Newman–Keuls test.
* Minor fatty acids made content up to 100 %.
Discussion
In the present study, dietary chia seed was used instead of maize oil (rich in LA) as the dietary source of fat to test whether or not this nutritional intervention could prevent and/or improve the dyslipidaemia and insulin insensitivity induced in rats fed a SRD. The major new findings in the present study are the following:
(1) Dietary chia seed was capable of preventing the onset of dyslipidaemia, when it was simultaneously administered with the SRD during 3 weeks (short-term experiment). The addition of chia seed did not change plasma glucose levels, and prevented the development of insulin resistance. Also, the significant increase of insulinaemia observed in the SRD-fed rats was also partially corrected.
(2) The well-established hypertriacylglycerolaemia and insulin resistance resulting from a long-term (5 months) feeding of normal rats with a SRD could be completely reversed despite the fact that no changes occurred in the circulating insulin levels when chia seed replaced the maize oil present in the rat diet during the last 2 months of the experimental period. Moreover, dietary chia seed decreased the visceral adiposity present in the SRD-fed rats without any significant changes in total body weight or energy intake.
As previously shown elsewhere(Reference Lombardo and Chicco6) and now confirmed in the present study, the substantial hypertriacylglycerolaemia which develops in rats fed a SRD, either during 3 weeks or 5 months, is a combination of an increased hepatic VLDL-TAG secretion and a defective removal mechanism of TAG from the circulation.
Moreover, several studies have demonstrated that a high-carbohydrate diet (sucrose or fructose) induces a significant increase of liver TAG content by increased lipogenic enzyme activities as well as by decreasing the activities of fatty acid oxidation(Reference Lombardo and Chicco6, Reference Lombardo, Hein and Chicco34, Reference Nagai, Nishio, Nakamura, Maegawa, Kikkawa and Kashiwagi35). The mechanisms involved in these processes include the down-regulation of PPARα and the increase of the gene expression of sterol regulatory element-binding protein-1 (SREBP-1)(Reference Nagai, Nishio, Nakamura, Maegawa, Kikkawa and Kashiwagi35).
It has been shown that long-chain n-3 PUFA (especially EPA and DHA) exhibit hypolipidaemic effects by coordinately suppressing hepatic lipid synthesis and secretion while inducing hepatic and skeletal muscle fatty acid oxidation(Reference Clarke2, Reference Lombardo and Chicco6, Reference Kim, Takahashi and Ezaki36). The n-3 PUFA, ALA, the major fatty acid present in the chia seed, could be converted to long-chain n-3 PUFA (mainly to EPA) in the liver. The effectiveness of this bioconversion can determine its effect on plasma lipids. Both the absolute amount of dietary ALA and the ratio of dietary LA:ALA influence the ALA conversion to EPA as a result of the known competition between n-6 and n-3 fatty acids for desaturation, since LA reduces Δ6 desaturase levels(Reference Benatti, Peluso, Nicolai and Calvani37). Jeffery et al. (Reference Jeffery, Sanderson, Sherringtton, Newsholme and Calder38) showed that the maximum hypotriacylglycerolaemic and hypocholesterolaemic effects in rats were observed with a LA:ALA ratio of 0·33, suggesting that the effect of ALA may be due to an increase of long-chain n-3 PUFA in membrane lipids(Reference Ihara, Umekawa, Takahashi and Furuichi11, Reference Jeffery, Sanderson, Sherringtton, Newsholme and Calder38).
The present study shows that by replacing maize oil (LA:ALA ratio 68·2) with chia seed (LA:ALA ratio 0·287) in rats fed a SRD during 3 weeks it was possible to prevent the usual SRD-induced hypertriacylglycerolaemia. The mechanisms involved in this process seem to be a combination of both a significant increase of plasma TAG clearance, which reached values higher than those recorded in the control diet group, and a reduction of the VLDL-TGSR. Moreover, the decreased availability of plasma NEFA could also participate, among other mechanisms, to prevent the increase of TAG content in the liver of the rats fed chia seed. A similar picture emerges after a long-term SRD feeding period. In this dietary group, hypertriacylglycerolaemia and both VLDL-TAG secretion and plasma TAG clearance were completely normalised by shifting the source of fat in the diet from maize oil to chia seed during the last 2 months of the feeding period. Kim et al. (Reference Kim, Choi and Choi12) also demonstrated that the administration of perilla oil (rich in ALA) to normal rats was effective in reducing plasma and hepatic TAG levels. In spite of the twofold decreasing plasma NEFA, the liver TAG content in the SRD-fed rats, although significantly diminished by the addition of chia seed, still remained high when compared with values recorded in the control diet-fed rats. However, the rats fed the long-term SRD showed extremely high liver TAG content before the addition of chia seed. Under these particular circumstances, we may assume that the time period of chia seed intake may not have been long enough to completely normalise the liver TAG contents. Ide(Reference Ide39) demonstrated, in normal rats, that dietary ALA significantly increased the mRNA levels of several hepatic fatty acid oxidation enzymes. ALA proved to be a preferred substrate for β oxidation and thus it was shown to reduce TAG synthesis, decrease assembly and secretion of TAG-rich lipoprotein in the liver, decreasing their plasma levels(Reference Ide, Murata and Sugano40). Moreover, in the liver the mRNA expression and enzymic activity of the lipogenic enzyme fatty acid synthase was inhibited in rats fed perilla oil(Reference Kabir and Ide41). Besides, the addition of chia seed decreased plasma cholesterol levels and increased the HDL-cholesterol:cholesterol ratio which reached values similar to those observed in the control diet groups. In this regard, Ayerza & Coates(Reference Ayerza and Coates17) showed a hypocholesterolaemic effect when normal rats were fed ground chia seed during 4 weeks. Moreover, Ihara et al. (Reference Ihara, Umekawa, Takahashi and Furuichi11) showed that ALA has a more potent serum cholesterol-lowering ability than LA. The expression of hydroxymethyl glutaryl CoA reductase was lower in rats fed perilla oil than in those fed safflower-seed oil. A possible mechanism of the action of ALA in plasma cholesterol has been suggested to be via its conversion to EPA in the body(Reference Bravo, Ortu, Cantafora, Lambert, Avella, Mayes and Botham42). Also, the high percentage of soluble fibre content in the whole chia seed could also play a role in lowering plasma cholesterol levels since the hypocholesterolaemic effect of dietary fibre in rats(Reference Li, Kaneko, Qing, Wang, Wang and Sato43) and other rodents(Reference Martinez-Flores, Chang, Martinez-Bustos and Sgarbieri44) has been reported. Thus, both the conversion of ALA to EPA as well as the higher soluble fibre content in the chia seed diet could be possible mechanisms involved in the reduction of hypercholesterolaemia in rats fed a SRD.
Previous studies have demonstrated that diets rich in EPA and DHA prevent excessive visceral adipose tissue growth in rats fed either a high-fat or a high-sucrose diet(Reference Lombardo and Chicco6). In the present studies, the visceral adiposity was observed only in the rats fed the SRD for a long term and was significantly reduced when chia seed replaced maize oil as the source of dietary fat. Since the food intake was similar to that of the SRD-fed rats, this effect was unlikely to be related to energy differences. Similarly, Okuno et al. (Reference Okuno, Kajiwara, Imai, Kobayashi, Honma, Maki, Suruga, Goda, Takase, Muto and Moriwaki45) indicated a reduction of white fat pad mass, in part by suppressing the late phase of adipocyte differentiation in rats fed perilla oil during 4 months compared with those fed saturated fats or LA-rich-fat diets. However, Ghafoorunissa et al. (Reference Ghafoorunissa, Ibrahim and Natarajan27) showed in rats fed a SRD for 3 months that the substitution of one-third of dietary LA with ALA did not reduce visceral fat weight. Difference in the n-6:n-3 ratio present in the diet (0·287 in the present study v. 2 in Ghafoorunissa et al. (Reference Ghafoorunissa, Ibrahim and Natarajan27)) may contribute to the effectiveness of chia seed in improving visceral adiposity.
Confirming previous results, plasma glucose and insulin levels evolved from normoglycaemia and hyperinsulinaemia after a short-term (3–5 weeks) consumption of the SRD to moderate hyperglycaemia and normoinsulinaemia after a long-term one (from 3 up to 8 months). While in both stages a whole-body peripheral insulin resistance was present, in the latter period insulin sensitivity further deteriorated and it was accompanied by a significant increase in the intramyocellular TAG content in the skeletal muscle. In this regard, studies in rats and human subjects have shown that the degree of insulin resistance is strongly correlated with the local accumulation of TAG and long-chain acyl CoA in this tissue(Reference Chicco, D'Alessandro, Karabatas, Pastorale, Basabe and Lombardo25, Reference Bronwyn, Poynten, Lowry, Furler, Chisholm, Kraegen and Cooney46). The administration of chia seed prevented the development of peripheral insulin resistance and partially decreased hyperinsulinaemia in the rats fed the SRD during 3 weeks. Furthermore, the impaired glucose homeostasis and peripheral insulin insensitivity were completely normalised in the rats fed the SRD during 5 months when chia seed was added for the last 2 months of the experimental period. Moreover, the TAG content within the skeletal muscle returned to normal values after the addition of chia seed. Thus, in the present study, the enhancement of peripheral insulin sensitivity may be largely a result of the significant action of chia seed to decrease circulating lipids and enhance whole-body glucose metabolism. In this regard, long-chain n-3 PUFA, which significantly reduce plasma lipid levels, have been shown to prevent and/or normalise peripheral insulin resistance in both high-sucrose- and high-fat-fed rats(Reference Lombardo and Chicco6). On the other hand, the relative and absolute amounts of LA and ALA in the diet and the competitive interactions in the metabolism of LA and ALA to long-chain n-6 and n-3 PUFA affect the membrane lipid composition. This, in turn, moderates a wide range of eicosanoid- and non-eicosanoid-mediated effects upon the metabolic process and could directly affect insulin action. In this regard, Ghafoorunissa et al. (Reference Ghafoorunissa, Ibrahim and Natarajan27) recently demonstrated a high level of long-chain n-3 PUFA in skeletal muscle and adipocyte plasma membrane phospholipids in rats fed for 3 months a SRD containing a LA:ALA ratio of 2. Interestingly, similar to the present results, the lipid-lowering effect of ALA in this study was accompanied by an increase in peripheral insulin sensitivity in both skeletal muscle and adipose tissue. Besides, rats fed dietary perilla oil containing neither EPA nor DHA resulted in high contents of both fatty acids in hepatic membranes with a low content of arachidonic acid(Reference Kim and Choi13). Furthermore, the present study shows an increase of plasma long-chain n-3 PUFA levels in the rats fed the SRD containing chia seed as compared with rats fed maize oil at equal energy levels. Similar results were reported by Ayerza & Coates(Reference Ayerza and Coates17) in rats fed a control diet with ground chia seed. Thus, although in the present study we have not quantified the fatty acid composition of skeletal muscle phospholipids, it is possible that changes in their composition after chia seed administration also contribute to the normalisation of peripheral insulin resistance.
In brief, the present study provides new data regarding the beneficial effect of chia seed (rich in ALA, fibre and minerals) upon lipid and glucose homeostasis in our experimental model of dyslipidaemia and insulin resistance. Caution is warranted before extrapolating these results to humans, since the overall efficiency of conversion of dietary ALA to EPA, docosapentaenoic acid and DHA is very low (for a review, see Burdge & Calder(Reference Burdge and Calder47)). However, Vuksan et al. (Reference Vuksan, Whitham, Sievenpiper, Jenkins, Rogovik, Bazinet, Vidgen and Hanna48) have recently demonstrated that long-term supplementation with Salba (S. hispanica L.) attenuates major (systolic blood pressure) and emerging cardiovascular risk factors safely beyond conventional therapy, while maintaining good glycaemic and lipid control in individuals with well-controlled type 2 diabetes.
Acknowledgements
The present study was carried out with the financial support of the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) and CONICET, grants PICT 05-38 157 BID 1728 OC/AR, 2005, PIP no. 5619/2005 and CAI+D 2-11 2006.
The authors thank Agrisalba SA, Buenos Aires, Argentina for providing the chia seed Salba.
A. G. C. was involved in the analysis of the euglycaemic–hyperinsulinaemic clamp and contributed to the discussion of the manuscript. M. E. D. was involved in the analysis of VLDL-TGSR and the intravenous fat tolerance test of a fat emulsion. G. J. H. was involved in the analysis of serum fatty acid composition and collaboration in the euglycaemic–hyperinsulinaemic clamp studies. M. E. O. was involved in the determination of the analytical methods for cholesterol, HDL-cholesterol, TAG, glucose and immunoreactive insulin. Y. B. L. wrote the manuscript and discussed it with the whole group of authors.
There are no conflicts of interest among members of the group.












