Food allergies are responsible for the considerable rise in morbidity and, in some cases, mortality. There are concerns that the incidence, prevalence and severity of food allergies are increasing in many parts of the world, particularly in children( Reference Ben-Shoshan, Turnbull and Clarke 1 – Reference Bock, Munoz-Furlong and Sampson 3 ). Food allergies are associated with significant reductions in the quality of life of both the affected individuals and their family members, which lead to a combination of the restrictive lifestyle associated with living with food allergy, the often considerable difficulties in avoiding the responsible food allergens and the potential for the occurrence of sudden life-threatening anaphylactic reactions( Reference Gupta, Sheikh and Strachan 4 , Reference Primeau, Kagan and Joseph 5 ).
Until now, the cornerstones of the clinical management of food allergies have been the identification and complete avoidance of the responsible food allergen(s)( 6 , Reference Otsu and Fleischer 7 ) and, in those who have had severe reactions, the carriage and use of self-injectable epinephrine (adrenaline). This management strategy is challenging, requiring considerable vigilance to avoid accidental exposure( Reference Simons, Ardusso and Bilo 8 , Reference Gallagher, Worth and Cunningham-Burley 9 ). In contrast to meticulous allergen avoidance, immunotherapy is the deliberate controlled exposure of patients with food allergy to extremely low, but progressively increasing doses of the offending allergen over a period of weeks or months( Reference Burks, Calderon and Casale 10 ). The aim is to reduce immunological sensitivity to the allergen such that patients can safely consume food containing the allergen or, at the very least, not react to an accidental low-dose exposure. This approach has, for example, over the last century become an established clinical practice in relation to the treatment of severe pollen, insect venom and drug allergies. Although the first case report of successful immunotherapy to food allergies was reported over a 100 years ago( Reference Schofield 11 ), this treatment is yet to become established in the management of people with food allergy. The increasing numbers of people living with potentially life-threatening food allergies and the preventable loss of life from food- triggered anaphylaxis have stimulated renewed interest in the role of orally administered immunotherapy – i.e. via the oral and sublingual routes – in the management of people with food allergy. This is particularly true for patients/parents of affected children who have been heartened by the widespread media coverage of a ‘cure’ for food allergies, but who also often express frustration that this has not been translated into clinical practice yet. In order to inform ongoing scientific and clinical deliberations on the role of orally administered immunotherapy, in the present study, we sought to critically assess the evidence on the effectiveness, mechanisms and safety of this potentially disease-modifying treatment approach( Reference Shenassa, Perelmutter and Gerrard 12 – Reference Fisher, du Toit and Lack 20 ).
Methods
Literature search and study selection
We searched for randomised controlled trials, quasi-randomised controlled trials and controlled clinical trials investigating the role of oral immunotherapy (OIT) and sublingual immunotherapy (SLIT) in children and adults with IgE-mediated (i.e. immediate hypersensitivity) food allergy. Our primary outcomes of interest were recovery rate from food allergy as assessed by the ability to consume the offending food allergen while receiving treatment (i.e. desensitisation) and, in particular, success rates for the ability to consume the food safely after completion of treatment (i.e. tolerance). Secondary outcomes of interest were immunological changes; the frequency and degree of local (i.e. minor oropharyngeal/gastrointestinal) and systematic (i.e. urticaria, angio-oedema, asthma and anaphylaxis) adverse events during treatment; quality of life; health service utilisation including emergency hospital admissions and emergency treatments; and data on costs from the perspective of health services.
For this purpose, we searched eleven international databases for published material: Cochrane Library; MEDLINE; EMBASE; LILACS; ISI Web of Science; BIOSIS; Global Health; AMED; TRIP; CAB; CINAHL (for search terms used, see Appendix 1, available online). In addition, we searched Internet-based international trial repositories such as www.clinicaltrials.gov and www.controlled-trials.com and contacted international experts in order to locate unpublished and ongoing work (see Appendix 2, available online). Our database searches covered the period from January 1990 to March 2013. The bibliographies of all eligible studies were scrutinised to identify additional possible studies. No language restrictions were imposed, and where necessary, manuscripts were translated into English.
Data abstraction
The titles and abstracts of the identified studies were checked and independently reviewed by two researchers (U. N. and G. D.). The full text of all the potentially eligible studies was assessed for eligibility against the eligibility criteria. Data were independently abstracted by two reviewers onto a customised data extraction sheet. Any disagreements were resolved through discussion, with A. S. arbitrating if an agreement could not be reached.
Quality assessment
The methodological quality of the included randomised controlled trials and quasi-randomised controlled trials was independently assessed using the methods detailed in section eight of the Cochrane Handbook for Systematic Reviews of Interventions ( 21 ). Critical appraisal of the controlled clinical trials was undertaken using the Cochrane Effective Practice and Organisation of Care (EPOC) guidelines( 22 ). We concentrated on using the following six parameters to assess quality: adequate sequence generation; allocation concealment; blinding/patient-related outcomes; the addressing of incomplete outcome data; the absence of selective reporting and the absence of other sources of bias. Each parameter of trial quality was graded: A – low risk of bias; B – moderate risk of bias; C – high risk of bias, and an overall assessment of quality for each trial using these three categories was carried out through consensus discussion among the reviewers.
Data synthesis
The clinical and statistical appropriateness of meta-analyses was considered for all outcomes of interest. Because of the clinical heterogeneity of the populations and interventions studied, we carried out a meta-analysis using random-effects modelling using Review Manager 5.1( 21 , Reference Riley, Higgins and Deeks 23 ). We calculated mean differences as continuous outcomes and risk ratios (RR) with 95 % CI. Because of a lack of consistency in the reporting of immunological outcomes (e.g. skin prick test, IgE and IgG4), original data were obtained from the authors of several trials. A priori sensitivity analyses were undertaken by study design and quality to assess the robustness of findings and explain any heterogeneity uncovered; where possible, subgroup analyses were undertaken on the basis of OIT and SLIT and the allergy being treated for. We graphically assessed for the possibility of publication bias using funnel plots.
Results
Our searches identified 1152 potentially relevant papers, from which we identified twenty-one trials (reported in twenty-two papers) that satisfied our inclusion criteria (Fig. 1). There were eighteen randomised controlled trials( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 – Reference Varshney, Jones and Scurlock 38 ) and three controlled clinical trials( Reference Patriarca, Nucera and Pollastrini 15 , Reference Mansouri, Movahhedi and Pourpak 39 , Reference Patriarca, Nucera and Roncallo 40 ) (Table 1). Of these trials, seventeen had investigated OIT( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 , Reference Caminiti, Passalacqua and Barberi 25 , Reference Lacono, Tripodi and Calvani 30 – Reference Patriarca, Nucera and Roncallo 40 ) and four had investigated SLIT( Reference Enrique, Pineda and Malek 26 – Reference Kim, Bird and Kulis 29 ). There was one report that included two independent randomised controlled trials on cows' milk and hens' eggs( Reference Morisset, Moneret-Vautrin and Guenard 34 ). Apart from these, twelve studies had focused on cows' milk( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Skripak, Nash and Rowley 18 , Reference Caminiti, Passalacqua and Barberi 25 , Reference Longo, Barbi and Berti 31 , Reference Martorell, De la Hoz and Ibanez 32 , Reference Morisset, Moneret-Vautrin and Guenard 34 , Reference Salmivesi, Korppi and Makela 37 , Reference Mansouri, Movahhedi and Pourpak 39 , Reference Patriarca, Nucera and Roncallo 40 ), eight on hens' eggs( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Burks, Jones and Wood 24 , Reference Lacono, Tripodi and Calvani 30 , Reference Meglio, Giampietro and Carello 33 , Reference Morisset, Moneret-Vautrin and Guenard 34 , Reference Patriarca, Schiavino and Nucera 36 , Reference Patriarca, Nucera and Roncallo 40 ), four on peanut( Reference Fleischer, Burks and Vickery 28 , Reference Kim, Bird and Kulis 29 , Reference Varshney, Jones and Scurlock 38 , Reference Patriarca, Nucera and Roncallo 40 ) and five other studies on a variety of food allergens including hazelnut( Reference Enrique, Pineda and Malek 26 ), peach( Reference Fernandez-Rivas, Garrido and Nadal 27 ), orange( Reference Patriarca, Nucera and Roncallo 40 ), apple( Reference Patriarca, Nucera and Pollastrini 15 , Reference Patriarca, Schiavino and Nucera 36 , Reference Patriarca, Nucera and Roncallo 40 ), ‘corn’( Reference Patriarca, Nucera and Roncallo 40 ), fish( Reference Patriarca, Nucera and Pollastrini 15 , Reference Patriarca, Schiavino and Nucera 36 , Reference Patriarca, Nucera and Roncallo 40 ), bean( Reference Patriarca, Nucera and Pollastrini 15 , Reference Patriarca, Nucera and Roncallo 40 ), wheat( Reference Patriarca, Nucera and Pollastrini 15 ) and lettuce( Reference Patriarca, Nucera and Roncallo 40 ) (see Appendix 3, available online). There were two follow-up studies( Reference Enrique, Pineda and Bartra 41 , Reference Garcia, Gonzales-Mancebo and Barber 42 ), and these focused on SLIT for hazelnut( Reference Enrique, Pineda and Malek 26 ) and peach allergies( Reference Fernandez-Rivas, Garrido and Nadal 27 ). Translation was required for two papers( Reference Mansouri, Movahhedi and Pourpak 39 , Reference Kurihara 43 ). Among the trials, sixteen had conducted studies on only children( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Burks, Jones and Wood 24 , Reference Caminiti, Passalacqua and Barberi 25 , Reference Kim, Bird and Kulis 29 – Reference Mansouri, Movahhedi and Pourpak 39 ), two on only adults( Reference Enrique, Pineda and Malek 26 , Reference Fernandez-Rivas, Garrido and Nadal 27 ) and three on both children and adults( Reference Skripak, Nash and Rowley 18 , Reference Fleischer, Burks and Vickery 28 , Reference Patriarca, Nucera and Roncallo 40 ).

Fig. 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. RCT, randomised controlled trial; CCT, controlled clinical trial; OIT, oral immunotherapy; SLIT, sublingual immunotherapy. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Table 1 Description of the included studies (n 21)

HSU, health service utilisation; OIT, oral immunotherapy; SLIT, sublingual immunotherapy; SPT, skin prick test; SBPCFC, single-blind placebo-controlled food challenge; DBPCFC, double-blind placebo-controlled food challenge; QOL, quality of life; LR, local reactions; SR, systemic reactions; Sp IgE, specific IgE, RCT, randomised controlled trial; CCT, controlled clinical trial.
* Other includes orange, maize, bean and lettuce.
† Other includes IL-4, IL-5, IL-10, IL-13, tumour growth factor β, interferon-γ, basophil activation and T regulatory cells.
‡ Follow-up study.
§ Cows' milk RCT.
∥ Hens' egg RCT.
Quality assessment
Quality assessment of these studies revealed that three of the randomised controlled trials were at a low risk of bias( Reference Fleischer, Burks and Vickery 28 , Reference Longo, Barbi and Berti 31 , Reference Varshney, Jones and Scurlock 38 ), a further five randomised controlled trials( Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 , Reference Fernandez-Rivas, Garrido and Nadal 27 , Reference Kim, Bird and Kulis 29 , Reference Martorell, De la Hoz and Ibanez 32 ) were judged to be at a moderate risk of bias and the remaining ten randomised controlled trials and the three controlled clinical trials( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Caminiti, Passalacqua and Barberi 25 , Reference Enrique, Pineda and Malek 26 , Reference Lacono, Tripodi and Calvani 30 , Reference Meglio, Giampietro and Carello 33 – Reference Salmivesi, Korppi and Makela 37 , Reference Mansouri, Movahhedi and Pourpak 39 , Reference Patriarca, Nucera and Roncallo 40 ) were all judged to be at a high risk of bias (see Appendix 4 for further details, available online).
Impact on primary outcomes
Desensitisation
The effectiveness of immunotherapy was compared with that of placebo with food avoidance/strict elimination diet( Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 – Reference Kim, Bird and Kulis 29 , Reference Longo, Barbi and Berti 31 , Reference Salmivesi, Korppi and Makela 37 , Reference Varshney, Jones and Scurlock 38 ) or food avoidance/strict elimination diet alone( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Lacono, Tripodi and Calvani 30 , Reference Martorell, De la Hoz and Ibanez 32 – Reference Morisset, Moneret-Vautrin and Guenard 34 , Reference Patriarca, Schiavino and Nucera 36 , Reference Mansouri, Movahhedi and Pourpak 39 , Reference Patriarca, Nucera and Roncallo 40 ). In two studies( Reference Pajno, Caminiti and Ruggeri 35 , Reference Salmivesi, Korppi and Makela 37 ) that had investigated the effectiveness of OIT for cows' milk allergy, soya milk was used as the control. A meta-analysis of the risk of persisting food allergy at the completion of the intervention period as assessed by a double-blind placebo-controlled food challenge was possible based on data obtained from all the twenty trials, which revealed a substantially reduced average risk of persisting food allergy in treated patients (RR 0·21, 95 % CI 0·12, 0·38; Fig. 2(a)) ( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 – Reference Patriarca, Schiavino and Nucera 36 , Reference Varshney, Jones and Scurlock 38 – Reference Patriarca, Nucera and Roncallo 40 ). A sensitivity analysis omitting the studies that had utilised a clinical diagnosis of food allergy (well-documented reaction within 60 min of consuming food and elevated specific IgE levels and/or a positive skin prick test) as an inclusion criterion instead of a confirmatory double-blind placebo-controlled food challenge made little difference to the summary estimates (RR 0·26, 95 % CI 0·15, 0·45) (see Appendix 5, Supplementary Fig. S1, available online)
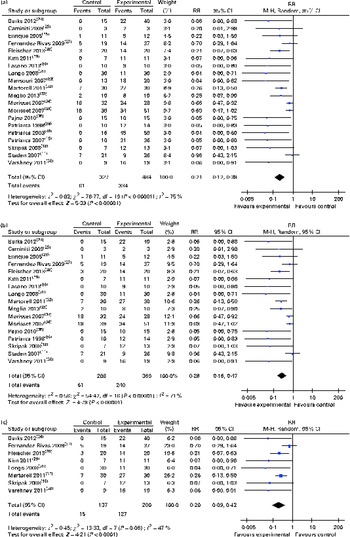
Fig. 2 (a) Risk ratios (RR) of persisting food allergy as assessed by double-blind placebo-controlled food challenge in oral immunotherapy (OIT) or sublingual immunotherapy (SLIT) v. controls, (b) sensitivity analysis RR of food allergy after OIT or SLIT (only randomised controlled trial) and (c) sensitivity analysis RR of food allergy after OIT or SLIT (only grade A and B studies). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Sensitivity analysis of the seventeen randomised controlled trials found a comparable average risk reduction (RR 0·28, 95 % CI 0·16, 0·47; Fig. 2(b)). Further sensitivity analysis excluding all the trials judged to be at a high risk of bias also demonstrated a substantial average risk reduction (RR 0·20, 95 % CI 0·09, 0·42)( Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 , Reference Fernandez-Rivas, Garrido and Nadal 27 – Reference Kim, Bird and Kulis 29 , Reference Longo, Barbi and Berti 31 , Reference Martorell, De la Hoz and Ibanez 32 , Reference Varshney, Jones and Scurlock 38 ) (Fig. 2(c)).
Subgroup analyses revealed that both oral (RR 0·19, 95 % CI 0·09, 0·37) and sublingual approaches had comparable effectiveness (RR 0·30, 95 % CI 0·12, 0·78) (Figs. 3 and 4, respectively).

Fig. 3 Risk ratios (RR) of persisting food allergy as assessed by double-blind placebo-controlled food challenge in oral immunotherapy v. controls. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
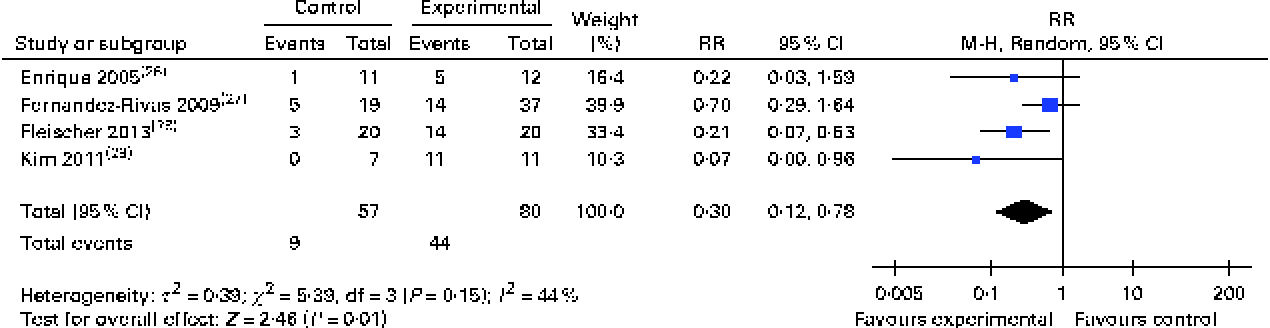
Fig. 4 Risk ratios (RR) of persisting food allergy as assessed by double-blind placebo-controlled food challenge in sublingual immunotherapy v. controls. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Furthermore, we were able to carry out subgroup analyses for eight trials that had investigated immunotherapy for cows' milk allergy, four trials on hens' egg allergy and three trials on peanut allergy. These analyses demonstrated that OIT approaches substantially reduced the risk of cows' milk (RR 0·14, 95 % CI 0·04, 0·44)( Reference Skripak, Nash and Rowley 18 , Reference Caminiti, Passalacqua and Barberi 25 , Reference Longo, Barbi and Berti 31 , Reference Martorell, De la Hoz and Ibanez 32 , Reference Morisset, Moneret-Vautrin and Guenard 34 – Reference Patriarca, Schiavino and Nucera 36 , Reference Mansouri, Movahhedi and Pourpak 39 ), hens' egg (RR 0·19, 95 % CI 0·04, 0·99)( Reference Burks, Jones and Wood 24 , Reference Lacono, Tripodi and Calvani 30 , Reference Meglio, Giampietro and Carello 33 , Reference Morisset, Moneret-Vautrin and Guenard 34 ) and peanut (RR 0·16, 95 % CI 0·06, 0·41)( Reference Fleischer, Burks and Vickery 28 , Reference Kim, Bird and Kulis 29 , Reference Varshney, Jones and Scurlock 38 ) allergies (see Appendix 5, Supplementary Figs. S2, S3 and S4, available online).
There was no clear evidence of publication bias (Fig. 5).
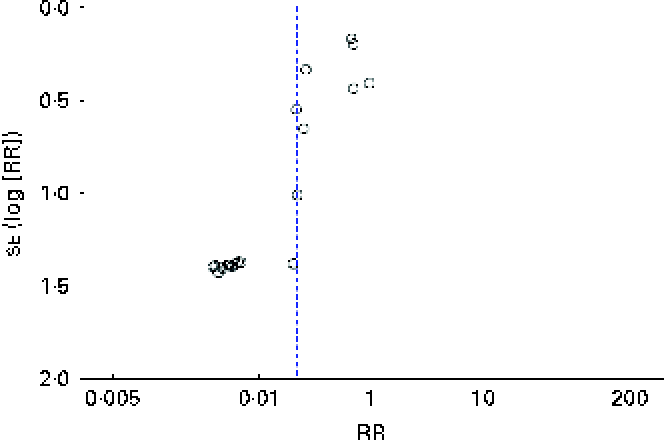
Fig. 5 Funnel plot showing: risk ratios (RR) of persistent food allergy after oral or sublingual immunotherapy. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Tolerance
Long-term tolerance was investigated by two studies, with it being studied after OIT in children with allergy to cows' milk and hens' eggs( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Burks, Jones and Wood 24 ). After completion of the desensitisation and maintenance phases, the subjects were subjected to a 1- to 2-month strict elimination (washout) period before the follow-up double-blind placebo-controlled food challenge. Burks et al. ( Reference Burks, Jones and Wood 24 ) reported that of the forty children undergoing hens' egg OIT, eleven (28 %) were considered to have sustained unresponsiveness after cessation of OIT (i.e. tolerance). Staden et al. ( Reference Staden, Rolinck-Werninghaus and Brewe 14 ) reported that there was no difference in the development of long-term tolerance between OIT and control subjects (35 v. 36 %), suggesting that regular allergen exposure was required to maintain the state of desensitisation.
Impact on secondary outcomes
Immunological outcomes
Many of the trials included data on the effects of OIT or SLIT on immunological outcomes (Appendices 6 and 7, available online). Skin prick test responses to the responsible food allergen before and after immunotherapy were measured by fifteen studies( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 , Reference Fernandez-Rivas, Garrido and Nadal 27 – Reference Lacono, Tripodi and Calvani 30 , Reference Martorell, De la Hoz and Ibanez 32 – Reference Morisset, Moneret-Vautrin and Guenard 34 , Reference Varshney, Jones and Scurlock 38 – Reference Patriarca, Nucera and Roncallo 40 ), food allergen-specific IgE levels by eighteen studies( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 , Reference Enrique, Pineda and Malek 26 – Reference Martorell, De la Hoz and Ibanez 32 , Reference Morisset, Moneret-Vautrin and Guenard 34 – Reference Patriarca, Schiavino and Nucera 36 , Reference Varshney, Jones and Scurlock 38 – Reference Patriarca, Nucera and Roncallo 40 ) and food allergen-specific IgG4 levels by eleven studies( Reference Patriarca, Nucera and Pollastrini 15 , Reference Skripak, Nash and Rowley 18 , Reference Burks, Jones and Wood 24 , Reference Enrique, Pineda and Malek 26 – Reference Kim, Bird and Kulis 29 , Reference Meglio, Giampietro and Carello 33 , Reference Pajno, Caminiti and Ruggeri 35 , Reference Varshney, Jones and Scurlock 38 , Reference Patriarca, Nucera and Roncallo 40 ).
Allergen skin prick tests
The results of allergen skin prick tests were expressed in differing formats. However, we were able to conduct a meta-analysis of skin prick test data obtained from five studies using a combination of published data and original data supplied by the investigators. OIT/SLIT reduced the magnitude of the mean wheal diameter response to the responsible food allergen by − 2·96 (95 % CI − 4·48, − 1·45) mm (Fig. 6), and of the ten studies that had failed to provide us with original data( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Longo, Barbi and Berti 31 , Reference Morisset, Moneret-Vautrin and Guenard 34 , Reference Patriarca, Schiavino and Nucera 36 ), eight( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Burks, Jones and Wood 24 , Reference Enrique, Pineda and Malek 26 , Reference Lacono, Tripodi and Calvani 30 – Reference Martorell, De la Hoz and Ibanez 32 , Reference Pajno, Caminiti and Ruggeri 35 ) reported that OIT/SLIT reduced skin prick test reactivity, with three studies reporting no change( Reference Fleischer, Burks and Vickery 28 , Reference Meglio, Giampietro and Carello 33 , Reference Pajno, Caminiti and Ruggeri 35 ). Subgroup analysis of data showed that OIT for cows' milk allergy also reduced the magnitude of the mean wheal diameter response to cows' milk by − 3·42 (95 % CI − 6·18, − 0·66) mm (see Appendix 5, Supplementary Fig. S5, available online).
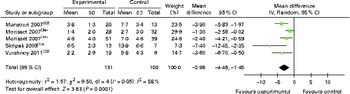
Fig. 6 Skin prick test (wheal in mm) following oral immunotherapy for food allergy. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Food allergen-specific IgE tests
The results of food allergen-specific IgE tests were expressed in differing formats, but we were able to conduct a meta-analysis of food allergen-specific IgE data obtained from six studies using published data and original data supplied by the investigators. Completion of OIT/SLIT did not significantly reduce the allergen-specific IgE levels ( − 5·2 (95 % CI − 12·3, 1·99) kU/l; Fig. 7). Of the studies that had failed to provide us with original data and not included in the meta-analysis, four( Reference Burks, Jones and Wood 24 , Reference Fernandez-Rivas, Garrido and Nadal 27 , Reference Fleischer, Burks and Vickery 28 , Reference Pajno, Caminiti and Ruggeri 35 ) reported that orally administered immunotherapy did not change the allergen-specific IgE levels and seven( Reference Staden, Rolinck-Werninghaus and Brewe 14 , Reference Patriarca, Nucera and Pollastrini 15 , Reference Kim, Bird and Kulis 29 , Reference Lacono, Tripodi and Calvani 30 , Reference Martorell, De la Hoz and Ibanez 32 , Reference Meglio, Giampietro and Carello 33 , Reference Patriarca, Nucera and Roncallo 40 ) reported that OIT/SLIT reduced their levels. Subgroup analysis of data showed that OIT also did not significantly reduce these levels ( − 8·96 for cows' milk allergy, 95 % CI − 28·64, 10·73; see Appendix 5, Supplementary Fig. S6, available online).

Fig. 7 Specific IgE levels (kU/l) following oral immunotherapy for food allergy. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Food allergen-specific IgG4 tests
The results of food allergen-specific IgG4 tests were expressed in differing formats, but we were able to conduct a meta-analysis of allergen-specific IgG4 data obtained from three studies using published data and original data supplied by the investigators. OIT/SLIT increased the allergen-specific IgG4 levels by 19·9 (95 % CI 17·1, 22·6) μg/ml (Fig. 8), and five of the seven studies that had failed to provide us with original data and not included in the meta-analysis also reported increases in their levels( Reference Patriarca, Nucera and Pollastrini 15 , Reference Burks, Jones and Wood 24 , Reference Fernandez-Rivas, Garrido and Nadal 27 , Reference Kim, Bird and Kulis 29 , Reference Patriarca, Nucera and Roncallo 40 ) and two studies( Reference Fleischer, Burks and Vickery 28 , Reference Meglio, Giampietro and Carello 33 ) reported no changes. Subgroup analysis of food allergen-specific IgG4 levels during OIT for cows' milk allergy also showed an increase in their levels (19·8 (95 % CI 14·32, 25·34) μg/ml; see Appendix 5, Supplementary Fig. S7, available online).
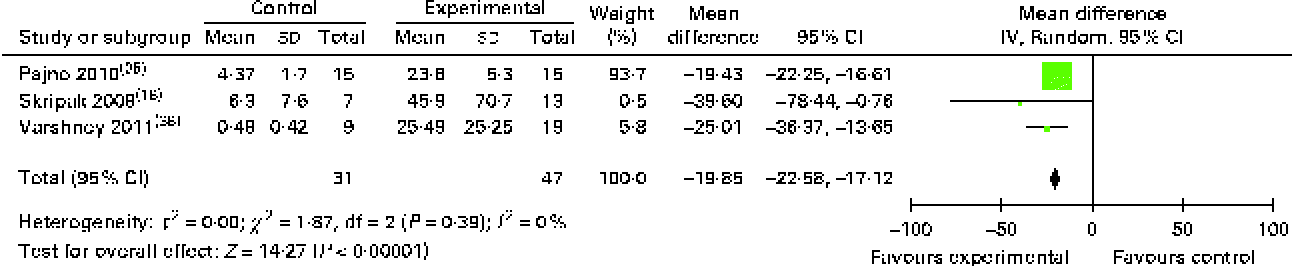
Fig. 8 IgG4 levels (μg/ml) following oral immunotherapy for food allergy. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Safety
Systemic reactions
Data on the occurrence of systemic (i.e. urticaria, asthma and anaphylaxis) adverse reactions were available from five trials. Meta-analysis of safety data obtained from these trials indicated a modest increased risk of systemic adverse reactions associated with treatment, but this was imprecisely estimated (RR 1·08, 95 % CI 0·97, 1·19)( Reference Enrique, Pineda and Malek 26 , Reference Fernandez-Rivas, Garrido and Nadal 27 , Reference Pajno, Caminiti and Ruggeri 35 , Reference Patriarca, Schiavino and Nucera 36 , Reference Varshney, Jones and Scurlock 38 , Reference Mansouri, Movahhedi and Pourpak 39 ) (Fig. 9). Some studies reported no ‘severe’ side effects( Reference Burks, Jones and Wood 24 , Reference Lacono, Tripodi and Calvani 30 , Reference Martorell, De la Hoz and Ibanez 32 , Reference Meglio, Giampietro and Carello 33 ). Focusing on only higher-quality studies (i.e. grade A and B studies) in a sensitivity analysis produced comparable summary estimates of the risk of adverse events (RR 1·02, 95 % CI 0·89, 1·17) (see Appendix 5, Supplementary Fig. S8, available online)( Reference Fernandez-Rivas, Garrido and Nadal 27 , Reference Varshney, Jones and Scurlock 38 ). However, subgroup analysis of safety data obtained from OIT studies( Reference Pajno, Caminiti and Ruggeri 35 , Reference Patriarca, Schiavino and Nucera 36 , Reference Mansouri, Movahhedi and Pourpak 39 ) for cows' milk allergy more clearly demonstrated these increased risks (RR 1·23, 95 % CI 1·03, 1·48; see Appendix 5, Supplementary Fig. S9, available online).

Fig. 9 Safety data – absence of systemic reactions during oral immunotherapy or sublingual immunotherapy for food allergy. RR, risk ratio. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Local reactions
Data on the occurrence of local (minor oropharyngeal/gastrointestinal) adverse reactions were available from nine studies; these revealed an increased risk associated with OIT/SLIT (RR 1·47, 95 % CI 1·11, 1·95) (Fig. 10). Studies not included in the meta-analysis reported the incidence of local reactions in relation to doses administered, indicating that OIT was associated with an increase in local reactions( Reference Burks, Jones and Wood 24 , Reference Fleischer, Burks and Vickery 28 , Reference Lacono, Tripodi and Calvani 30 ). Including only grade A and B studies in a sensitivity analysis demonstrated a small non-significant increased risk of local reactions associated with immunotherapy (RR 2·08, 95 % CI 0·87, 4·99; see Appendix 5, Supplementary Fig. S10, available online)( Reference Burks, Jones and Wood 24 , Reference Fernandez-Rivas, Garrido and Nadal 27 , Reference Martorell, De la Hoz and Ibanez 32 , Reference Varshney, Jones and Scurlock 38 ). Subgroup analysis of data obtained from trials on OIT for cows' milk allergy suggested an increased risk in the treatment arm, but this was imprecisely estimated (RR 2·03, 95 % CI 0·87, 4·73; see Appendix 5, Supplementary Fig. S11, available online).

Fig. 10 Safety data – absence of local reactions during oral immunotherapy or sublingual immunotherapy for food allergy. RR, risk ratio. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Other outcomes
None of the studies had reported on the other outcomes of interest, namely quality of life of patients and their families; use of health services including emergency hospital admissions and emergency treatments; and data on cost-effectiveness considerations.
Details of unpublished and ongoing studies are summarised in Appendix 8 (available online).
Discussion
Statement of principal findings
The present systematic review and meta-analysis has found that orally administered immunotherapy is likely to be effective in substantially reducing the risk of persisting food allergy in children and adults with IgE-mediated food allergy to a range of foods while receiving treatment (i.e. desensitisation was successfully achieved). The increases in allergen exposure that people are able to tolerate while on treatment are clinically relevant and are likely to prevent many of the reactions associated with accidental exposure. It remains unclear as to whether orally administered immunotherapy induces clinical tolerance (i.e. long-term cure). For example, Burks et al. ( Reference Burks, Jones and Wood 24 ) reported that OIT induced tolerance in 28 % of those treated, whereas Staden et al. ( Reference Staden, Rolinck-Werninghaus and Brewe 14 ) found no increase in tolerance over and above that observed in the control subjects. The lack of consensus on clinical tolerance is important because of the need for regular exposure to allergenic foods to maintain a state of desensitisation. These treated patients, therefore, at present need to move from a situation in which they are meticulously avoiding the food in question to a state in which regular consumption of the food is necessary in order to maintain a desensitised state. Such a state of desensitisation may be associated with improved quality of life; however, the psychological consequences (if any) of such a radical change in management strategy may in some individuals adversely affect the quality of life. These issues need to be addressed by appropriate trials. Immunotherapy is associated with an increased risk of local side effects and, more importantly, may also be associated with a modest increased risk of systemic side effects, necessitating very careful intensive monitoring of patients and high-level clinical support (i.e. access to specialist advice 24 h a day, 7 d a week). The cost implications for health services of treating immunotherapy-associated adverse events, the supervision of immunotherapy dose increases in clinical areas and the provision of high-level clinical support have not been addressed by any of the studies identified and also clearly need further investigation.
Insights into the mechanisms of action
In contrast to previous reviews on this subject( Reference Fisher, du Toit and Lack 20 , Reference Kurihara 43 – Reference Brożek, Terracciano and Hsu 49 ), we also studied and synthesised data on immunological outcomes. Overall, the immunological data suggest that orally administered immunotherapy induces changes in skin prick tests (reduced response) and antigen-specific IgG4 levels (increased) similar to those reported with conventional allergen immunotherapy and during the natural early-life development of tolerance to food allergens( Reference Akdis and Akdis 50 ). The majority of the studies reported that orally administered immunotherapy did not reduce allergen-specific IgE levels, and this was confirmed by the meta-analysis. The disparity in the ability of orally administered immunotherapy to reduce skin prick test reactivity to the responsible allergens while failing to reduce serum allergen-specific IgE levels may be a consequence of increased levels of allergen-specific IgG4 inhibiting IgE cross-linking by competing with IgE for the binding of allergens( Reference Strait, Morris and Finkelman 51 ). It is also possible that reduced skin prick test reactivity may be a consequence of the effects of orally administered immunotherapy on non-IgE components of the skin prick test, e.g. mast cells, or possibly the generation of IgE with a reduced binding affinity for the allergens.
Strengths and weaknesses of this work
We believe that this is the most comprehensive and detailed systematic review and meta-analysis on this subject ever undertaken. This work has been conducted to international standards and, furthermore, has both drawn on a substantially greater evidence base and has considerable methodological strengths over previous reviews on this subject( Reference Fisher, du Toit and Lack 20 , Reference Kurihara 43 – Reference Brożek, Terracciano and Hsu 49 ). It provides a state-of-the-art overview of the experimental evidence on this clinically important subject together with detailed subgroup/sensitivity analyses based on allergy to specific foods, mode of immunotherapy and study design. The quality assessment acknowledged the inherent weakness of uncontrolled trials in young children with food allergy, whereby food allergies in early life naturally resolve as tolerance develops, e.g. cows' milk allergy.
The main potential limitations of this work stem from the heterogeneity of the populations, interventions and outcomes studied/reported on; it is, therefore, important that, in keeping with the random-effects meta-analyses employed, care be taken in interpreting the findings as average effects across studies. That said, our various subgroup and sensitivity analyses, with accompanying reductions in heterogeneity in some cases (see Fig. 2(b) and (c), Appendix 5, Figs. S1 and S4, available online), generated broadly comparable findings, which suggests that the overall conclusions are very likely to be robust. Although we found that orally administered immunotherapy is associated with an increased likelihood of relatively mild local side effects, because of inconsistencies in the definition and reporting, our meta-analyses of side effects were limited to a minority of studies and to a handful of studies at a low risk of bias. Clearly, further trials using standardised reporting of side effects are required to fully assess the risks associated with orally administered immunotherapy. A further limitation is the failure of some investigators to provide us with original data; however, the reported effects of immunotherapy in these studies are consistent with the results of our meta-analyses. Future studies also need to determine longer-term outcomes, as most studies to date have been short-term ones with less than 2 years of follow-up. Finally, we have uncovered data on ongoing studies, the findings of which will, once incorporated into our planned updates of this systematic review and meta-analysis, offer greater precision around the summary estimates.
Implications for clinical care and further research
In summary, orally administered immunotherapy for IgE-mediated food allergy is a promising re-emerging treatment approach, which has the potential to play an important disease-modifying role in people with a range of food allergies. Current treatment regimens are, however, associated with an increased risk of local reactions and possibly also more serious systemic reactions; therefore, orally administered immunotherapy is not suitable for use in routine clinical care and should not under any circumstances be considered as a self-administered treatment approach. There is a pressing need to develop safer treatment protocols and establish the longer-term effectiveness, safety and cost-effectiveness of this potentially curative treatment approach.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513002353
Acknowledgements
The present study was funded by the Chief Scientist's Office (CSO) of the Scottish Government CZG/2/493. The authors' contributions are as follows: A. S. conceived the present study and together with U. N. and G. D. secured the funding; U. N. and G. D. undertook the searches and together with A. S. critically appraised the studies; U. N., G. D., L. H. and A. W. were responsible for data extraction, with U. N. and A. S. leading the analysis; U. N. and A. S. drafted the manuscript. All authors commented on the drafts of the paper. The authors have no conflicts of interest to declare.















